A positively charged cluster in the epidermal growth factor-like domain of Factor VII-activating protease (FSAP) is essential for polyanion binding
- PMID: 16332249
- PMCID: PMC1383718
- DOI: 10.1042/BJ20051563
A positively charged cluster in the epidermal growth factor-like domain of Factor VII-activating protease (FSAP) is essential for polyanion binding
Abstract
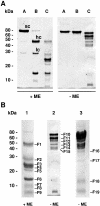
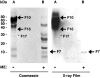
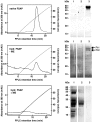
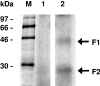
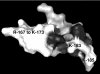
FSAP (Factor VII-activating protease) is a novel plasma-derived serine protease that regulates haemostasis as well as vascular cell proliferation. FSAP undergoes autoactivation in the presence of polyanionic macromolecules such as heparin and RNA. Competition experiments suggest that RNA and heparin bind to the same or overlapping interaction sites. A proteolysis approach, where FSAP was hydrolysed into smaller fragments, was used to identify the polyanion-binding site. The EGF (epidermal growth factor)-like domains EGF2 and EGF3 of FSAP are the major interaction domains for RNA. The amino acids Arg170, Arg171, Ser172 and Lys173 within the EGF3 domain were essential for this binding. This is also the region with the highest positive net charge in the protein and is most probably located in an exposed loop. It is also highly conserved across five species. Disruption of disulphide bridges led to the loss of RNA and heparin binding, indicating that the three-dimensional structure of the EGF3 domain is essential for binding to negatively charged heparin or RNA. The identification of polyanion-binding sites will help to define the role of FSAP in the vasculature.
Figures


References
-
- Kannemeier C., Feussner A., Stöhr H. A., Weisse J., Preissner K. T., Römisch J. Factor VII and single-chain plasminogen activator-activating protease: activation and autoactivation of the proenzyme. Eur. J. Biochem. 2001;268:3789–3796. - PubMed
-
- Römisch J. Factor VII activating protease (FSAP): a novel protease in hemostasis. Biol. Chem. 2002;383:1119–1124. - PubMed
-
- Choi-Miura N. H., Tobe T., Sumiya J. I., Nakano Y., Sano Y., Mazda T., Tomita M. Purification and characterization of a novel hyaluronan-binding protein (PHBP) from human plasma: it has three EGF, a kringle and a serine protease domain, similar to hepatocyte growth factor activator. J. Biochem. (Tokyo) 1996;119:1157–1165. - PubMed
-
- Sumiya J. I., Asakawa S., Tobe T., Hashimoto K., Saguchi K. I., Choi-Miura N. H., Shimizu Y., Minoshima S., Shimizu N., Tomita M. Isolation and characterization of the plasma hyaluronan-binding protein (PHBP) gene (HABP2) J. Biochem. (Tokyo) 1997;122:983–990. - PubMed
-
- Etscheid M., Hunfeld A., Konig H., Seitz R., Dodt J. Activation of proPHBSP, the zymogen of a plasma hyaluronan binding serine protease, by an intermolecular autocatalytic mechanism. Biol. Chem. 2000;381:1223–1231. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

