Planktonic-cell yield of a pseudomonad biofilm
- PMID: 16332753
- PMCID: PMC1317325
- DOI: 10.1128/AEM.71.12.7792-7798.2005
Planktonic-cell yield of a pseudomonad biofilm
Abstract
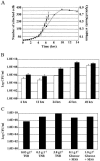
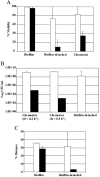
Biofilm cells differ phenotypically from their free-floating counterparts. Differential growth rates in biofilms are often referred to, particularly in response to limited diffusion of oxygen and nutrients. We observed growth rates of attached Pseudomonas sp. strain CT07 cells that were notably higher than the maximum specific growth rate measured in batch culture. Despite dilution rates in continuous flow cells that exceeded the maximum planktonic specific growth rate by 58 times, sampling of the effluent revealed >10(9) cells ml(-1), suggesting that biofilms function as a source of planktonic cells through high cell yield and detachment. Further investigation demonstrated considerable planktonic cell yield from biofilms as young as 6 h, indicating that detachment is not limited to established biofilms. These biofilm-detached cells were more sensitive to a commercial biocide than associated biofilm- and chemostat-cultivated populations, implying that detached biofilm cells exhibit a character that is distinct from that of attached and planktonic cell populations.
Figures

References
-
- Andrews, J. H., and R. F. Harris. 1986. r- and K-selection and microbial ecology. In K. C. Marshall (ed.), Advances in microbial ecology, vol. 9. Kluwer Academic/Plenum Publishers, New York, N.Y.
-
- Atlas, R. M., and R. Bartha. 1998. Microbial ecology: fundamentals and applications, 4th ed. Benjamin/Cummins Science Publishing, Menlo Park, Calif.
-
- Barton, A. J., R. D. Sagers, and W. G. Pitt. 1996. Measurement of bacterial growth rates on polymers. J. Biomed. Mat. Res. 32:271-278. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

