Amplified expression of fructose 1,6-bisphosphatase in Corynebacterium glutamicum increases in vivo flux through the pentose phosphate pathway and lysine production on different carbon sources
- PMID: 16332851
- PMCID: PMC1317465
- DOI: 10.1128/AEM.71.12.8587-8596.2005
Amplified expression of fructose 1,6-bisphosphatase in Corynebacterium glutamicum increases in vivo flux through the pentose phosphate pathway and lysine production on different carbon sources
Abstract
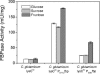
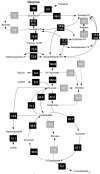
The overexpression of fructose 1,6-bisphosphatase (FBPase) in Corynebacterium glutamicum leads to significant improvement of lysine production on different sugars. Amplified expression of FBPase via the promoter of the gene encoding elongation factor TU (EFTU) increased the lysine yield in the feedback-deregulated lysine-producing strain C. glutamicum lysCfbr by 40% on glucose and 30% on fructose or sucrose. Additionally formation of the by-products glycerol and dihydroxyacetone was significantly reduced in the PEFTUfbp mutant. As revealed by 13C metabolic flux analysis on glucose the overexpression of FBPase causes a redirection of carbon flux from glycolysis toward the pentose phosphate pathway (PPP) and thus leads to increased NADPH supply. Normalized to an uptake flux of glucose of 100%, the relative flux into the PPP was 56% for C. glutamicum lysCfbr PEFTUfbp and 46% for C. glutamicum lysCfbr. The flux for NADPH supply was 180% in the PEFTUfbp strain and only 146% in the parent strain. Amplification of FBPase increases the production of lysine via an increased supply of NADPH. Comparative studies with another mutant containing the sod promoter upstream of the fbp gene indicate that the expression level of FBPase relates to the extent of the metabolic effects. The overexpression of FBPase seems useful for starch- and molasses-based industrial lysine production with C. glutamicum. The redirection of flux toward the PPP should also be interesting for the production of other NADPH-demanding compounds as well as for products directly stemming from the PPP.
Figures

References
-
- Abbouni, B., H. M. Elhariry, and G. Auling. 2004. Overproduction of NAD+ and 5′-inosine monophosphate in the presence of 10 μM Mn2+ by a mutant of Corynebacterium ammoniagenes with thermosensitive nucleotide reduction (nrd(ts)) after temperature shift. Arch. Microbiol. 182:119-125. - PubMed
-
- Babul, J., and V. Guixe. 1983. Fructose bisphosphatase from Escherichia coli. Purification and characterization. Arch. Biochem. Biophys. 225:944-949. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
-
- Dominguez, H., C. Rollin, A. Guyonvarch, J. L. Guerquin-Kern, M. Cocaign-Bousquet, and N. D. Lindley. 1998. Carbon-flux distribution in the central metabolic pathways of Corynebacterium glutamicum during growth on fructose. Eur. J. Biochem. 254:96-102. - PubMed
-
- Gamo, F. J., M. A. Navas, M. A. Blazquez, C. Gancedo, and J. M. Gancedo. 1994. Catabolite inactivation of heterologous fructose-1,6-bisphosphatases and fructose-1,6-bisphosphatase-beta-galactosidase fusion proteins in Saccharomyces cerevisiae. Eur. J. Biochem. 222:879-884. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

