Deficiency of LKB1 in heart prevents ischemia-mediated activation of AMPKalpha2 but not AMPKalpha1
- PMID: 16332922
- PMCID: PMC2128705
- DOI: 10.1152/ajpendo.00443.2005
Deficiency of LKB1 in heart prevents ischemia-mediated activation of AMPKalpha2 but not AMPKalpha1
Abstract
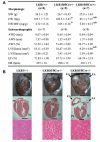
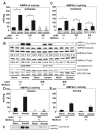
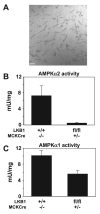
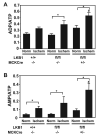
Recent studies indicate that the LKB1 is a key regulator of the AMP-activated protein kinase (AMPK), which plays a crucial role in protecting cardiac muscle from damage during ischemia. We have employed mice that lack LKB1 in cardiac and skeletal muscle and studied how this affected the activity of cardiac AMPKalpha1/alpha2 under normoxic, ischemic, and anoxic conditions. In the heart lacking cardiac muscle LKB1, the basal activity of AMPKalpha2 was vastly reduced and not increased by ischemia or anoxia. Phosphorylation of AMPKalpha2 at the site of LKB1 phosphorylation (Thr172) or phosphorylation of acetyl-CoA carboxylase-2, a downstream substrate of AMPK, was ablated in ischemic heart lacking cardiac LKB1. Ischemia was found to increase the ADP-to-ATP (ADP/ATP) and AMP-to-ATP ratios (AMP/ATP) to a greater extent in LKB1-deficient cardiac muscle than in LKB1-expressing muscle. In contrast to AMPKalpha2, significant basal activity of AMPKalpha1 was observed in the lysates from the hearts lacking cardiac muscle LKB1, as well as in cardiomyocytes that had been isolated from these hearts. In the heart lacking cardiac LKB1, ischemia or anoxia induced a marked activation and phosphorylation of AMPKalpha1, to a level that was only moderately lower than observed in LKB1-expressing heart. Echocardiographic and morphological analysis of the cardiac LKB1-deficient hearts indicated that these hearts were not overtly dysfunctional, despite possessing a reduced weight and enlarged atria. These findings indicate that LKB1 plays a crucial role in regulating AMPKalpha2 activation and acetyl-CoA carboxylase-2 phosphorylation and also regulating cellular energy levels in response to ischemia. They also provide genetic evidence that an alternative upstream kinase can activate AMPKalpha1 in cardiac muscle.
Figures

References
-
- Altarejos JY, Taniguchi M, Clanachan AS, Lopaschuk GD. Myocardial ischemia differentially regulates LKB1 and an alternate 5′-AMP-activated protein kinase kinase. J Biol Chem. 2005;280:183–190. - PubMed
-
- Anderson KA, Means RL, Huang QH, Kemp BE, Goldstein EG, Selbert MA, Edelman AM, Fremeau RT, Means AR. Components of a calmodulin-dependent protein kinase cascade. Molecular cloning, functional characterization and cellular localization of Ca2+/calmodulin-dependent protein kinase kinase beta. J Biol Chem. 1998;273:31880–31889. - PubMed
-
- Baron SJ, J Li, Russell RR, Neumann D, Miller EJ, Tuerk R, Wallimann T, Hurley RL, Witters LA, Young LH. Dual mechanisms regulating AMPK kinase action in the ischemic heart. Circ Res. 2005;96:337–345. - PubMed
-
- Beauloye C, Marsin AS, Bertrand L, Krause U, Hardie DG, Vanoverschelde JL, Hue L. Insulin antagonizes AMP-activated protein kinase activation by ischemia or anoxia in rat hearts, without affecting total adenine nucleotides. FEBS Lett. 2001;505:348–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- S18744/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 059528/Z/99/Z/JMW/CP/JF/WT_/Wellcome Trust/United Kingdom
- PG/04/086/17410/BHF_/British Heart Foundation/United Kingdom
- MC_U127070193/MRC_/Medical Research Council/United Kingdom
- G0400608/MRC_/Medical Research Council/United Kingdom
- C15048/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- PG/04/096/17627/BHF_/British Heart Foundation/United Kingdom
- MC_U127088492/MRC_/Medical Research Council/United Kingdom
- BREAST CANCER NOW RESEARCH CENTRE/BBC_/Breast Cancer Now/United Kingdom
- G0400608(71317)/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

