The latch-bridge hypothesis of smooth muscle contraction
- PMID: 16333357
- PMCID: PMC2278007
- DOI: 10.1139/y05-090
The latch-bridge hypothesis of smooth muscle contraction
Abstract
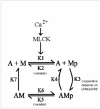
In contrast to striated muscle, both normalized force and shortening velocities are regulated functions of cross-bridge phosphorylation in smooth muscle. Physiologically this is manifested as relatively fast rates of contraction associated with transiently high levels of cross-bridge phosphorylation. In sustained contractions, Ca2+, cross-bridge phosphorylation, and ATP consumption rates fall, a phenomenon termed "latch". This review focuses on the Hai and Murphy (1988a) model that predicted the highly non-linear dependence of force on phosphorylation and a directly proportional dependence of shortening velocity on phosphorylation. This model hypothesized that (i) cross-bridge phosphorylation was obligatory for cross-bridge attachment, but also that (ii) dephosphorylation of an attached cross-bridge reduced its detachment rate. The resulting variety of cross-bridge cycles as predicted by the model could explain the observed dependencies of force and velocity on cross-bridge phosphorylation. New evidence supports modifications for more general applicability. First, myosin light chain phosphatase activity is regulated. Activation of myosin phosphatase is best demonstrated with inhibitory regulatory mechanisms acting via nitric oxide. The second modification of the model incorporates cooperativity in cross-bridge attachment to predict improved data on the dependence of force on phosphorylation. The molecular basis for cooperativity is unknown, but may involve thin filament proteins absent in striated muscle.
Figures



Similar articles
-
Regulation of shortening velocity by cross-bridge phosphorylation in smooth muscle.Am J Physiol. 1988 Jul;255(1 Pt 1):C86-94. doi: 10.1152/ajpcell.1988.255.1.C86. Am J Physiol. 1988. PMID: 3389402
-
An expanded latch-bridge model of protein kinase C-mediated smooth muscle contraction.J Appl Physiol (1985). 2005 Apr;98(4):1356-65. doi: 10.1152/japplphysiol.00834.2004. Epub 2004 Nov 19. J Appl Physiol (1985). 2005. PMID: 15557014
-
Cross-bridge phosphorylation and regulation of latch state in smooth muscle.Am J Physiol. 1988 Jan;254(1 Pt 1):C99-106. doi: 10.1152/ajpcell.1988.254.1.C99. Am J Physiol. 1988. PMID: 3337223
-
Inhibitory mechanisms for cross-bridge cycling: the nitric oxide-cGMP signal transduction pathway in smooth muscle relaxation.Acta Physiol Scand. 1998 Dec;164(4):373-80. doi: 10.1046/j.1365-201X.1998.00434.x. Acta Physiol Scand. 1998. PMID: 9887961 Review.
-
Regulation of contraction in striated muscle.Physiol Rev. 2000 Apr;80(2):853-924. doi: 10.1152/physrev.2000.80.2.853. Physiol Rev. 2000. PMID: 10747208 Review.
Cited by
-
Mechanisms underlying TNFα-induced enhancement of force generation in airway smooth muscle.Physiol Rep. 2019 Sep;7(17):e14220. doi: 10.14814/phy2.14220. Physiol Rep. 2019. PMID: 31512410 Free PMC article.
-
Regulation of Contraction by the Thick Filaments in Skeletal Muscle.Biophys J. 2017 Dec 19;113(12):2579-2594. doi: 10.1016/j.bpj.2017.09.037. Biophys J. 2017. PMID: 29262355 Free PMC article. Review.
-
Smooth muscle miRNAs are critical for post-natal regulation of blood pressure and vascular function.PLoS One. 2011 Apr 22;6(4):e18869. doi: 10.1371/journal.pone.0018869. PLoS One. 2011. PMID: 21526127 Free PMC article.
-
Force suppression and the crossbridge cycle in swine carotid artery.Am J Physiol Cell Physiol. 2007 Sep;293(3):C1003-9. doi: 10.1152/ajpcell.00091.2007. Epub 2007 May 23. Am J Physiol Cell Physiol. 2007. PMID: 17522140 Free PMC article.
-
Hypoxic pulmonary vasoconstriction.Physiol Rev. 2012 Jan;92(1):367-520. doi: 10.1152/physrev.00041.2010. Physiol Rev. 2012. PMID: 22298659 Free PMC article. Review.
References
-
- Adelstein RS, Sellers JR. Myosin structure and function. In: Bárány M, editor. Biochemistry of smooth muscle contraction. Academic Press; San Diego: 1996. pp. 3–19.
-
- Andersson K-E, Persson K. Nitric oxide synthase and the lower urinary tract: possible implications for physiology and pathophysiology. Scand. J. Urol. Nephrol. 1995;175:43–53. - PubMed
-
- Arner A, Goody RS, Rapp G, Rüegg JC. Relaxation of chemically skinned guinea pig taenia coli smooth muscle from rigor by photolytic release of adenosine-5′-triphosphate. J. Muscle. Res. Cell Motil. 1987;8:377–385. - PubMed
-
- Babu GJ, Warshaw DM, Periasamy M. Smooth muscle myosin heavy chain isoforms and their role in muscle physiology. Microscopy Res.Technique. 2000;50:532–540. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

