Heat shock protein 70, heat shock protein 32, and vascular endothelial growth factor production and their effects on lipopolysaccharide-induced apoptosis in porcine aortic endothelial cells
- PMID: 16333987
- PMCID: PMC1283877
- DOI: 10.1379/csc-98r1.1
Heat shock protein 70, heat shock protein 32, and vascular endothelial growth factor production and their effects on lipopolysaccharide-induced apoptosis in porcine aortic endothelial cells
Abstract
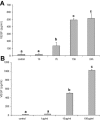
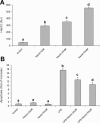
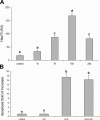
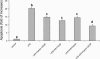
Lipopolysaccharide (LPS) is a highly proactive molecule that causes in vivo a systemic inflammatory response syndrome and activates in vitro the inflammatory pathway in different cellular types, including endothelial cells (EC). Because the proinflammatory status could lead to EC injury and apoptosis, the expression of proinflammatory genes must be finely regulated through the induction of protective genes. This study aimed at determining whether an LPS exposure is effective in inducing apoptosis in primary cultures of porcine aortic endothelial cells and in stimulating heat shock protein (Hsp)70 and Hsp32 production as well as vascular endothelial growth factor (VEGF) secretion. Cells between third and eighth passage were exposed to 10 microg/mL LPS for 1, 7, 15, and 24 hours (time-course experiments) or to 1, 10, and 100 microg/mL LPS for 7 and 15 hours (dose-response experiments). Apoptosis was not affected by 1 microg/mL LPS but significantly increased in a dose-dependent manner with the highest LPS doses. Furthermore, apoptosis rate increased only till 15 hours of LPS exposure. LPS stimulated VEGF secretion in a dose-dependent manner; its effect became significant after 7 hours and reached a plateau after 15 hours. Both Hsp70 and Hsp32 expressions were induced by LPS in a dose-dependent manner after 7 hours. Subsequent studies were addressed to evaluate the protective role of Hsp32, Hsp70, and VEGF. Hemin, an Hsp32 inducer (5, 20, 50 microM), and recombinant VEGF (100 and 200 ng/mL), were added to the culture 2 hours before LPS (10 microg/mL for 24 hours); to induce Hsp70 expression, cells were heat shocked (42 degrees C for 1 hour) 15 hours before LPS (10 microg/mL for 24 hours). Hemin exposure upregulated Hsp32 expression in a dose-dependent manner and protected cells against LPS-induced apoptosis. Heat shock (HS) stimulated Hsp70 expression but failed to reduce LPS-induced apoptosis; VEGF addition did not protect cells against LPS-induced apoptosis at any dose tested. Nevertheless, when treatments were associated, a reduction of LPS-induced apoptosis was always observed; the reduction was maximal when all the treatments (HS + Hemin + VEGF) were associated. In conclusion, this study demonstrates that LPS is effective in evoking "the heat shock response" with an increase of nonspecific protective molecules (namely Hsp70 and Hsp32) and of VEGF, a specific EC growth factor. The protective role of Hsp32 was also demonstrated. Further investigations are required to clarify the synergic effect of Hsp32, Hsp70, and VEGF, thus elucidating the possible interaction between these molecules.
Figures

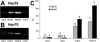
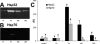
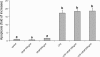
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
