The human skeletal alpha-actin gene is regulated by a muscle-specific enhancer that binds three nuclear factors
- PMID: 1633435
- PMCID: PMC6057388
The human skeletal alpha-actin gene is regulated by a muscle-specific enhancer that binds three nuclear factors
Abstract
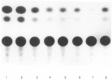
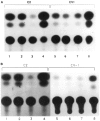
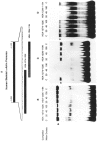
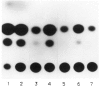
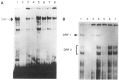
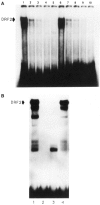
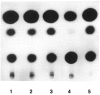
The tissue-specific distal promoter of the human skeletal alpha-actin gene (-1282 to -708) induces transcription in myogenic cells approximately 10-fold and, with the most proximal promoter domain (-153 to -87), it synergistically increases transcription 100-fold (Muscat and Kedes 1987). We report here that it is a short fragment of the distal promoter, the distal regulatory element (DRE) from -1282 to -1177 that functions as a muscle-specific, composite enhancer. An internal deletion in the DRE (delta -1282/-1151) in the context of the full-length 2000 bp promoter, resulted in a 10-fold reduction in transcription. Three distinct nuclear proteins, DRF-1, DRF-2, and DRF-3, interact specifically with the DRE between positions -1260 and -1193. A site specific mutation that abolishes DRF-2 binding also results in a 10-fold reduction in transcriptional activity. The DRF-2 nuclear protein has characteristics similar to those of the muscle-specific regulatory factor, MEF-2 (Buskin and Hauschka 1989; Gossett et al., 1989). Like the MEF-2 binding site in the muscle creatine kinase enhancer, the critical DRF-2 binding site is also an A/T-rich sequence element. The DRF-2 nuclear protein binds equally well to the MCK MEF-2 binding site and to the A/T-rich regulatory element of the skeletal muscle fast-twitch troponin C gene (Gahlmann and Kedes 1990). Furthermore, this troponin C site competes in vivo for DRF-2 driven expression of the skeletal alpha-actin gene in C2 cells. The DRF-2 site alone, however, does not activate transcription in muscle cells when linked to the SV40 promoter. We conclude that the DRF-2 binding element is a MEF-2 binding site that is required but insufficient for regulation of muscle-specific skeletal alpha-actin gene expression by the DRE. Thus, muscle-specific regulation of the human skeletal alpha-actin gene appears to require interactions between the other elements of the composite DRE enhancer with the protein:DNA complex formed by DRF-2.
Figures



References
-
- Bradford M. M. (1976), Anal Biochem 77, 248–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
