Cyclic nucleotide phosphodiesterases as targets for treatment of haematological malignancies
- PMID: 16336197
- PMCID: PMC1383661
- DOI: 10.1042/BJ20051368
Cyclic nucleotide phosphodiesterases as targets for treatment of haematological malignancies
Abstract
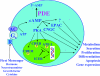
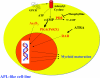
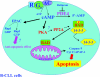
The cAMP signalling pathway has emerged as a key regulator of haematopoietic cell proliferation, differentiation and apoptosis. In parallel, general understanding of the biology of cyclic nucleotide PDEs (phosphodiesterases) has advanced considerably, revealing the remarkable complexity of this enzyme system that regulates the amplitude, kinetics and location of intracellular cAMP-mediated signalling. The development of therapeutic inhibitors of specific PDE gene families has resulted in a growing appreciation of the potential therapeutic application of PDE inhibitors to the treatment of immune-mediated illnesses and haematopoietic malignancies. This review summarizes the expression and function of PDEs in normal haematopoietic cells and the evidence that family-specific inhibitors will be therapeutically useful in myeloid and lymphoid malignancies.
Figures


References
-
- Rall T. W., Sutherland E. W. Formation of a cyclic adenine ribonucleotide by tissue particles. J. Biol. Chem. 1958;232:1065–1076. - PubMed
-
- Butcher R. W., Sutherland E. W. Adenosine 3′,5′-phosphate in biological materials. I. Purification and properties of cyclic 3′,5′-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3′,5′-phosphate in human urine. J. Biol. Chem. 1962;237:1244–1250. - PubMed
-
- Robison G. A., Butcher R. W., Oye I., Morgan H. E., Sutherland E. W. The effect of epinephrine on adenosine 3′,5′-phosphate levels in the isolated perfused rat heart. Mol. Pharmacol. 1965;1:168–177. - PubMed
-
- Pastan I. H., Johnson G. S., Anderson W. B. Role of cyclic nucleotides in growth control. Annu. Rev. Biochem. 1975;44:491–522. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

