TNFalpha- and IKKbeta-mediated TANK/I-TRAF phosphorylation: implications for interaction with NEMO/IKKgamma and NF-kappaB activation
- PMID: 16336209
- PMCID: PMC1383709
- DOI: 10.1042/BJ20051659
TNFalpha- and IKKbeta-mediated TANK/I-TRAF phosphorylation: implications for interaction with NEMO/IKKgamma and NF-kappaB activation
Abstract
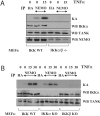
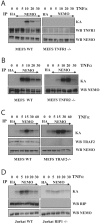
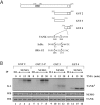
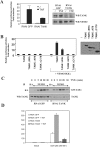
Pro-inflammatory cytokines trigger signalling cascades leading to NF-kappaB (nuclear factor-kappaB)-dependent gene expression through IKK [IkappaB (inhibitory kappaB) kinase]-dependent phosphorylation and subsequent degradation of the IkappaB proteins and via induced phosphorylation of p65. These signalling pathways rely on sequentially activated kinases which are assembled by essential and non-enzymatic scaffold proteins into functional complexes. Here, we show that the pro-inflammatory cytokine TNFalpha (tumour necrosis factor alpha) promotes TANK [TRAF (TNF receptor-associated factor) family member associated NF-kappaB activator] recruitment to the IKK complex via a newly characterized C-terminal zinc finger. Moreover, we show that TANK is phosphorylated by IKKbeta upon TNFalpha stimulation and that this modification negatively regulates TANK binding to NEMO (NF-kappaB essential modulator). Interestingly, reduced TANK expression by RNA interference attenuates TNFalpha-mediated induction of a subset of NF-kappaB target genes through decreased p65 transactivation potential. Therefore the scaffold protein TANK is required for the cellular response to TNFalpha by connecting upstream signalling molecules to the IKKs and p65, and its subsequent IKKbeta-mediated phosphorylation may be a mechanism to terminate the TANK-dependent wave of NF-kappaB activation.
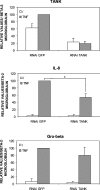
Figures



References
-
- Tracey K. J., Cerami A. Tumor necrosis factor, other cytokines and disease. Annu. Rev. Cell Biol. 1993;9:317–343. - PubMed
-
- Karin M., Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu. Rev. Immunol. 2000;18:621–663. - PubMed
-
- Hayden M. S., Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. - PubMed
-
- Hsu H., Xiong J., Goeddel D. V. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell (Cambridge, Mass.) 1995;81:495–504. - PubMed
-
- Devin A., Cook A., Lin Y., Rodriguez Y., Kelliher M., Liu Z. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity. 2000;12:419–429. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

