Modulation of NMDA receptors by pituitary adenylate cyclase activating peptide in CA1 neurons requires G alpha q, protein kinase C, and activation of Src
- PMID: 16339032
- PMCID: PMC6725893
- DOI: 10.1523/JNEUROSCI.3871-05.2005
Modulation of NMDA receptors by pituitary adenylate cyclase activating peptide in CA1 neurons requires G alpha q, protein kinase C, and activation of Src
Erratum in
- J Neurosci. 2005 Dec 14;25(50):table of contents
Abstract
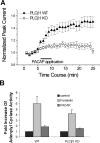
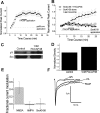
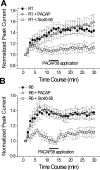
At CA1 synapses, activation of NMDA receptors (NMDARs) is required for the induction of both long-term potentiation and depression. The basal level of activity of these receptors is controlled by converging cell signals from G-protein-coupled receptors and receptor tyrosine kinases. Pituitary adenylate cyclase activating peptide (PACAP) is implicated in the regulation of synaptic plasticity because it enhances NMDAR responses by stimulating Galphas-coupled receptors and protein kinase A (Yaka et al., 2003). However, the major hippocampal PACAP1 receptor (PAC1R) also signals via Galphaq subunits and protein kinase C (PKC). In CA1 neurons, we showed that PACAP38 (1 nM) enhanced synaptic NMDA, and evoked NMDAR, currents in isolated CA1 neurons via activation of the PAC1R, Galphaq, and PKC. The signaling was blocked by intracellular applications of the Src inhibitory peptide Src(40-58). Immunoblots confirmed that PACAP38 biochemically activates Src. A Galphaq pathway is responsible for this Src-dependent PACAP enhancement because it was attenuated in mice lacking expression of phospholipase C beta1, it was blocked by preventing elevations in intracellular Ca2+, and it was eliminated by inhibiting either PKC or cell adhesion kinase beta [CAKbeta or Pyk2 (proline rich tyrosine kinase 2)]. Peptides that mimic the binding sites for either Fyn or Src on receptor for activated C kinase-1 (RACK1) also enhanced NMDAR in CA1 neurons, but their effects were blocked by Src(40-58), implying that Src is the ultimate regulator of NMDARs. RACK1 serves as a hub for PKC, Fyn, and Src and facilitates the regulation of basal NMDAR activity in CA1 hippocampal neurons.
Figures




References
-
- Alier KA, Morris BJ (2005) Divergent regulation of Pyk2/CAKbeta phosphorylation by Ca2+ and cAMP in the hippocampus. Biochim Biophys Acta - PubMed
-
- Arimura A, Somogyvari-Vigh A, Weill C, Fiore RC, Tatsuno I, Bay V, Brenneman DE (1994) PACAP functions as a neurotrophic factor. Ann NY Acad Sci 739: 228-243. - PubMed
-
- Chang BY, Chiang M, Cartwright CA (2001) The interaction of Src and RACK1 is enhanced by activation of protein kinase C and tyrosine phosphorylation of RACK1. J Biol Chem 276: 20346-20356. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
