Molecular and cellular diversity of neuronal G-protein-gated potassium channels
- PMID: 16339040
- PMCID: PMC6725904
- DOI: 10.1523/JNEUROSCI.3484-05.2005
Molecular and cellular diversity of neuronal G-protein-gated potassium channels
Abstract
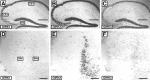
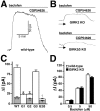
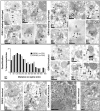
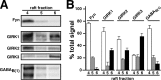
Neuronal G-protein-gated potassium (GIRK) channels mediate the inhibitory effects of many neurotransmitters. Although the overlapping distribution of GIRK subunits suggests that channel composition varies in the CNS, little direct evidence supports the existence of structural or functional diversity in the neuronal GIRK channel repertoire. Here we show that the GIRK channels linked to GABAB receptors differed in two neuron populations. In the substantia nigra, GIRK2 was the principal subunit, and it was found primarily in dendrites of neurons in the substantia nigra pars compacta (SNc). Baclofen evoked prominent barium-sensitive outward current in dopamine neurons of the SNc from wild-type mice, but this current was completely absent in neurons from GIRK2 knock-out mice. In the hippocampus, all three neuronal GIRK subunits were detected. The loss of GIRK1 or GIRK2 was correlated with equivalent, dramatic reductions in baclofen-evoked current in CA1 neurons. Virtually all of the barium-sensitive component of the baclofen-evoked current was eliminated with the ablation of both GIRK2 and GIRK3, indicating that channels containing GIRK3 contribute to the postsynaptic inhibitory effect of GABAB receptor activation. The impact of GIRK subunit ablation on baclofen-evoked current was consistent with observations that GIRK1, GIRK2, and GABAB receptors were enriched in lipid rafts isolated from mouse brain, whereas GIRK3 was found primarily in higher-density membrane fractions. Altogether, our data show that different GIRK channel subtypes can couple to GABAB receptors in vivo. Furthermore, subunit composition appears to specify interactions between GIRK channels and organizational elements involved in channel distribution and efficient receptor coupling.
Figures




References
-
- Alvarez FJ, Villalba RM, Zerda R, Schneider SP (2004) Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J Comp Neurol 472: 257-280. - PubMed
-
- Barr J, Van Bockstaele EJ (2005) Vesicular glutamate transporter-1 colocalizes with endogenous opioid peptides in axon terminals of the rat locus coeruleus. Anat Rec A Discov Mol Cell Evol Biol 284: 466-474. - PubMed
-
- Bartsch U, Bartsch S, Dorries U, Schachner M (1992) Immunohistological localization of tenascin in the developing and lesioned adult mouse optic nerve. Eur J Neurosci 4: 338-352. - PubMed
-
- Bettahi I, Marker CL, Roman MI, Wickman K (2002) Contribution of the Kir3.1 subunit to the muscarinic-gated atrial potassium channel IKACh. J Biol Chem 277: 48282-48288. - PubMed
-
- Blednov YA, Stoffel M, Chang SR, Harris RA (2001) GIRK2 deficient mice. Evidence for hyperactivity and reduced anxiety. Physiol Behav 74: 109-117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
