Conventional kinesin mediates microtubule-microtubule interactions in vivo
- PMID: 16339079
- PMCID: PMC1356599
- DOI: 10.1091/mbc.e05-06-0542
Conventional kinesin mediates microtubule-microtubule interactions in vivo
Abstract
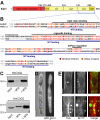
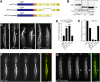
Conventional kinesin is a ubiquitous organelle transporter that moves cargo toward the plus-ends of microtubules. In addition, several in vitro studies indicated a role of conventional kinesin in cross-bridging and sliding microtubules, but in vivo evidence for such a role is missing. In this study, we show that conventional kinesin mediates microtubule-microtubule interactions in the model fungus Ustilago maydis. Live cell imaging and ultrastructural analysis of various mutants in Kin1 revealed that this kinesin-1 motor is required for efficient microtubule bundling and participates in microtubule bending in vivo. High levels of Kin1 led to increased microtubule bending, whereas a rigor-mutation in the motor head suppressed all microtubule motility and promoted strong microtubule bundling, indicating that kinesin can form cross-bridges between microtubules in living cells. This effect required a conserved region in the C terminus of Kin1, which was shown to bind microtubules in vitro. In addition, a fusion protein of yellow fluorescent protein and the Kin1tail localized to microtubule bundles, further supporting the idea that a conserved microtubule binding activity in the tail of conventional kinesins mediates microtubule-microtubule interactions in vivo.
Figures


Similar articles
-
Myosin-V, Kinesin-1, and Kinesin-3 cooperate in hyphal growth of the fungus Ustilago maydis.Mol Biol Cell. 2005 Nov;16(11):5191-201. doi: 10.1091/mbc.e05-04-0272. Epub 2005 Aug 24. Mol Biol Cell. 2005. PMID: 16120650 Free PMC article.
-
A balance of KIF1A-like kinesin and dynein organizes early endosomes in the fungus Ustilago maydis.EMBO J. 2002 Jun 17;21(12):2946-57. doi: 10.1093/emboj/cdf296. EMBO J. 2002. PMID: 12065408 Free PMC article.
-
Kinesin-3 in the basidiomycete Ustilago maydis transports organelles along the entire microtubule array.Fungal Genet Biol. 2015 Jan;74:59-61. doi: 10.1016/j.fgb.2014.10.010. Epub 2014 Oct 18. Fungal Genet Biol. 2015. PMID: 25459534
-
Tracks for traffic: microtubules in the plant pathogen Ustilago maydis.New Phytol. 2007;174(4):721-733. doi: 10.1111/j.1469-8137.2007.02072.x. New Phytol. 2007. PMID: 17504456 Review.
-
Lucky 13-microtubule depolymerisation by kinesin-13 motors.J Cell Sci. 2006 Oct 1;119(Pt 19):3905-13. doi: 10.1242/jcs.03224. J Cell Sci. 2006. PMID: 16988025 Review.
Cited by
-
Loukoumasomes Are Distinct Subcellular Structures from Rods and Rings and Are Structurally Associated with MAP2 and the Nuclear Envelope in Retinal Cells.PLoS One. 2016 Oct 31;11(10):e0165162. doi: 10.1371/journal.pone.0165162. eCollection 2016. PLoS One. 2016. PMID: 27798680 Free PMC article.
-
Cytoplasmic dynein crosslinks and slides anti-parallel microtubules using its two motor domains.Elife. 2013 Sep 3;2:e00943. doi: 10.7554/eLife.00943. Elife. 2013. PMID: 24015359 Free PMC article.
-
A pUL25 dimer interfaces the pseudorabies virus capsid and tegument.J Gen Virol. 2017 Nov;98(11):2837-2849. doi: 10.1099/jgv.0.000903. Epub 2017 Oct 16. J Gen Virol. 2017. PMID: 29035172 Free PMC article.
-
Dynein-dependent motility of microtubules and nucleation sites supports polarization of the tubulin array in the fungus Ustilago maydis.Mol Biol Cell. 2006 Jul;17(7):3242-53. doi: 10.1091/mbc.e05-12-1118. Epub 2006 May 3. Mol Biol Cell. 2006. PMID: 16672380 Free PMC article.
-
Enhanced Dynamics of Confined Cytoskeletal Filaments Driven by Asymmetric Motors.Biophys J. 2017 Sep 5;113(5):1121-1132. doi: 10.1016/j.bpj.2017.07.016. Biophys J. 2017. PMID: 28877494 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

