Cell proliferation depends on mitochondrial Ca2+ uptake: inhibition by salicylate
- PMID: 16339178
- PMCID: PMC1805645
- DOI: 10.1113/jphysiol.2005.100586
Cell proliferation depends on mitochondrial Ca2+ uptake: inhibition by salicylate
Abstract
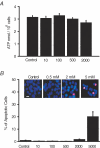
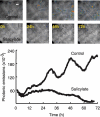
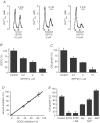
Store-operated Ca2+ entry (SOCE) is a ubiquitous Ca2+ influx pathway involved in control of multiple cellular and physiological processes including cell proliferation. Recent evidence has shown that SOCE depends critically on mitochondrial sinking of entering Ca2+ to avoid Ca2+-dependent inactivation. Thus, a role of mitochondria in control of cell proliferation could be anticipated. We show here that activation of SOCE induces cytosolic high [Ca2+] domains that are large enough to be sensed and avidly taken up by a pool of nearby mitochondria. Prevention of mitochondrial clearance of the entering Ca2+ inhibited both SOCE and cell proliferation in several cell types including Jurkat and human colon cancer cells. In addition, we find that therapeutic concentrations of salicylate, the major metabolite of aspirin, depolarize partially mitochondria and inhibit mitochondrial Ca2+ uptake, as revealed by mitochondrial Ca2+ measurements with targeted aequorins. This salicylate-induced inhibition of mitochondrial Ca2+ sinking prevented SOCE and impaired cell growth of Jurkat and human colon cancer cells. Finally, direct blockade of SOCE by the pyrazole derivative BTP-2 was sufficient to arrest cell growth. Taken together, our results reveal that cell proliferation depends critically on mitochondrial Ca2+ uptake and suggest that inhibition of tumour cell proliferation by salicylate may be due to interference with mitochondrial Ca2+ uptake, which is essential for sustaining SOCE. This novel mechanism may contribute to explaining the reported anti-proliferative and anti-tumoral actions of aspirin and dietary salicylates.
Figures
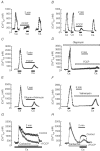
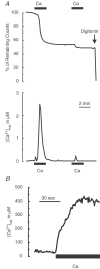


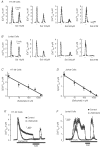
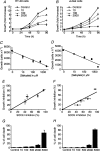

Similar articles
-
Nonsteroidal anti-inflammatory drugs inhibit vascular smooth muscle cell proliferation by enabling the Ca2+-dependent inactivation of calcium release-activated calcium/orai channels normally prevented by mitochondria.J Biol Chem. 2011 May 6;286(18):16186-96. doi: 10.1074/jbc.M110.198952. Epub 2011 Mar 14. J Biol Chem. 2011. PMID: 21402693 Free PMC article.
-
The role of mitochondrial potential in control of calcium signals involved in cell proliferation.Cell Calcium. 2008 Sep;44(3):259-69. doi: 10.1016/j.ceca.2007.12.002. Epub 2008 Feb 1. Cell Calcium. 2008. PMID: 18241916
-
Mitochondrial Ca2+ uptake and not mitochondrial motility is required for STIM1-Orai1-dependent store-operated Ca2+ entry.J Cell Sci. 2010 Aug 1;123(Pt 15):2553-64. doi: 10.1242/jcs.070151. Epub 2010 Jun 29. J Cell Sci. 2010. PMID: 20587595
-
Remodeling of Calcium Entry Pathways in Cancer.Adv Exp Med Biol. 2016;898:449-66. doi: 10.1007/978-3-319-26974-0_19. Adv Exp Med Biol. 2016. PMID: 27161240 Review.
-
Modulation of Calcium Entry by Mitochondria.Adv Exp Med Biol. 2016;898:405-21. doi: 10.1007/978-3-319-26974-0_17. Adv Exp Med Biol. 2016. PMID: 27161238 Review.
Cited by
-
Delphinidin Reduces Glucose Uptake in Mice Jejunal Tissue and Human Intestinal Cells Lines through FFA1/GPR40.Int J Mol Sci. 2017 Apr 5;18(4):750. doi: 10.3390/ijms18040750. Int J Mol Sci. 2017. PMID: 28379159 Free PMC article.
-
CBARA1 plays a role in stemness and proliferation of human embryonic stem cells.PLoS One. 2013 May 8;8(5):e63653. doi: 10.1371/journal.pone.0063653. Print 2013. PLoS One. 2013. PMID: 23667653 Free PMC article.
-
Hydrogen Sulfide-Evoked Intracellular Ca2+ Signals in Primary Cultures of Metastatic Colorectal Cancer Cells.Cancers (Basel). 2020 Nov 11;12(11):3338. doi: 10.3390/cancers12113338. Cancers (Basel). 2020. PMID: 33187307 Free PMC article.
-
Beyond a chemopreventive reagent, aspirin is a master regulator of the hallmarks of cancer.J Cancer Res Clin Oncol. 2019 Jun;145(6):1387-1403. doi: 10.1007/s00432-019-02902-6. Epub 2019 Apr 29. J Cancer Res Clin Oncol. 2019. PMID: 31037399 Free PMC article. Review.
-
Development of Store-Operated Calcium Entry-Targeted Compounds in Cancer.Front Pharmacol. 2021 May 28;12:688244. doi: 10.3389/fphar.2021.688244. eCollection 2021. Front Pharmacol. 2021. PMID: 34122115 Free PMC article. Review.
References
-
- Aceves M, Dueñas A, Gómez C, San Vicente E, Sánchez Crespo M, García-Rodríguez C. A new pharmacological effect of salicylates, inhibition of NFAT-dependent transcription. J Immunol. 2004;173:5721–5729. - PubMed
-
- Alvarez J, Montero M. Ca2+ measurements with luminescent proteins in the endoplasmic reticulum. In: Petersen OH, editor. Measuring Calcium and Calmodulin Inside and Outside Cells. Berlin: Springer Laboratory Manual, Springer; 2001. pp. 147–163.
-
- Alvarez J, Montero M, García-Sancho J. Agonist-induced Ca2+ influx in human neutrophils is not mediated by production of inositol polyphosphates but by emptying of the intracellular Ca2+ stores. Biochem Soc Transact. 1994;22:809–813. - PubMed
-
- Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–899. - PubMed
-
- Bernardi P. Mitochondrial transport of cations: channels, exchangers and permeability transition. Physiol Rev. 1999;79:1127–1155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

