Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial
- PMID: 16339220
- PMCID: PMC1325128
- DOI: 10.1136/bmj.38666.653600.55
Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial
Erratum in
- BMJ. 2006 Jan 21;332(7534):151
Abstract
Objective: To determine if the macrolide clarithromycin affects mortality and cardiovascular morbidity in patients with stable coronary heart disease.
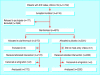
Design: Centrally randomised multicentre trial. All parties at all stages were blinded. Analyses were by intention to treat.
Setting: Five Copenhagen University cardiology departments and a coordinating centre.
Participants: 13,702 patients aged 18 to 85 years who had a discharge diagnosis of myocardial infarction or angina pectoris in 1993-9 and alive in August 1999 were invited by letter; 4373 were randomised.
Interventions: Two weeks' treatment with clarithromycin 500 mg/day or matching placebo.
Primary outcome: composite of all cause mortality, myocardial infarction, or unstable angina pectoris during three years' follow-up. Secondary outcome: composite of cardiovascular mortality, myocardial infarction, or unstable angina pectoris. The outcomes were obtained from Danish registers and were blindly assessed by the event committee.
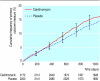
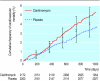
Results: 2172 participants were randomised to clarithromycin and 2201 to placebo. We found no significant effects of clarithromycin on the primary outcome (hazard ratio 1.15, 95% confidence interval 0.99 to 1.34) or secondary outcome (1.17, 0.98 to 1.40). Mortality was significantly higher in the clarithromycin arm (1.27, 1.03 to 1.54; P = 0.03) as a result of significantly higher cardiovascular mortality (1.45, 1.09 to 1.92; P = 0.01).
Conclusions: Short term clarithromycin in patients with stable coronary heart disease may cause significantly higher cardiovascular mortality. The long term safety of clarithromycin in patients with stable ischaemic heart disease should be examined. Trial registration ClinicalTrials.gov NCT00121550.
Figures
Republished in
-
[Intervention with clarithromycin in patients with stable coronary heart disease--the CLARICOR trial: secondary publication].Ugeskr Laeger. 2007 Feb 5;169(6):497-9. Ugeskr Laeger. 2007. PMID: 17303029 Danish.
Comment in
-
Clarithromycin may increase cardiovascular mortality in people with stable heart disease.Evid Based Cardiovasc Med. 2006 Jun;10(2):147-8. doi: 10.1016/j.ebcm.2006.04.023. Epub 2006 May 24. Evid Based Cardiovasc Med. 2006. PMID: 16753542 No abstract available.
References
-
- Libby P, Ridker PM, Maser A. Inflammation and atherosclerosis. Circulation 2002;105: 1135-43. - PubMed
-
- Muhlestein JB, Anderson JL. Infectious serology and atherosclerosis: how burdensome is the risk? Circulation 2003;107: 220-2. - PubMed
-
- Gelfand EV, Cannon CP. Antibiotics for secondary prevention of coronary artery disease: an ACES hypothesis but we need to PROVE IT. Am Heart J 2004;147: 202-9. - PubMed
-
- Gieffers J, Solbach W, Maass M. In vitro susceptibility and eradication of Chlamydia pneumoniae cardiovascular strains from coronary artery endothelium and smooth muscle cells. Cardiovasc Drugs Ther 2001;15: 259-62. - PubMed
-
- Melissano G, Blasi F, Esposito G, Tarsia P, Dordoni L, Arosio C, et al. Chlamydia pneumoniae eradication from carotid plaques: results of an open, randomised treatment study. Eur J Vasc Endovasc Surg 1999;18: 355-9. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
