Evidence for differential acquired drug resistance to anti-tumour necrosis factor agents in rheumatoid arthritis
- PMID: 16339288
- PMCID: PMC1798167
- DOI: 10.1136/ard.2005.045062
Evidence for differential acquired drug resistance to anti-tumour necrosis factor agents in rheumatoid arthritis
Abstract
Background: Acquired drug resistance or gradual drug failure has been described with most disease modifying antirheumatic drugs (DMARDs) and is also starting to be recognised with anti-tumour necrosis factor (anti-TNF) agents.
Objective: To study acquired drug resistance to anti-TNF agents in rheumatoid arthritis (RA).
Methods: Swiss health authorities requested continuous monitoring of patients receiving biological agents. Intensification of co-therapy with traditional DMARDs, gradual dose escalation, and drug discontinuation rates in all patients receiving infliximab, etanercept, or adalimumab, adjusting for potential confounders, were analysed. Intensification of DMARD co-therapy and time to discontinuation of the three anti-TNF agents were analysed using a proportional hazards models. Dose escalation and evolution of RA disease activity (DAS28) were analysed using a longitudinal regression model.
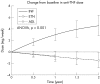
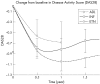
Results: 1198 patients contributing 1450 patient-years of anti-TNF treatment met the inclusion criteria. The rate of intensification of traditional DMARD co-therapy over time was significantly higher with infliximab (hazards ratio = 1.73 (99% confidence interval (CI) 1.19 to 2.51)) than with the two other agents. Infliximab also showed significant dose escalation over time, with an average dose increase of +12% (99% CI 8% to 16%) after 1 year, and +18% (99% CI 11% to 25%) after 2 years. No significant differences in discontinuation rates were seen between the three anti-TNF agents (ANOVA, p = 0.67). Evolution of disease activity over time indicated a lower therapeutic response to infliximab (DAS28, p<0.001) compared with etanercept, after 6 months' treatment.
Conclusions: In this population, infliximab was associated with a higher risk of requiring intensification of DMARD co-therapy than the other anti-TNF agents and a significant dose escalation over time. Analysis of RA disease activity indicated a reduced therapeutic response to infliximab after the first 6 months of treatment, suggestive of acquired drug resistance.
Comment in
-
Differential drug resistance to anti-tumour necrosis factor agents in rheumatoid arthritis.Ann Rheum Dis. 2006 Jun;65(6):701-3. doi: 10.1136/ard.2005.049890. Ann Rheum Dis. 2006. PMID: 16699049 Free PMC article.
References
-
- Weinblatt M E, Keystone E C, Furst D E, Moreland L W, Weisman M H, Birbara C A.et al Adalimumab, a fully human anti‐tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum 20034835–45. - PubMed
-
- Wallis R S, Broder M S, Wong J Y, Hanson M E, Beenhouwer D O. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis 2004381261–1265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous