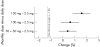Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the MOBILE study
- PMID: 16339289
- PMCID: PMC1798147
- DOI: 10.1136/ard.2005.044958
Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the MOBILE study
Erratum in
- Ann Rheum Dis. 2008 Feb;67(2):280
Abstract
Background: Reducing bisphosphonate dosing frequency may improve suboptimal adherence to treatment and therefore therapeutic outcomes in postmenopausal osteoporosis. Once-monthly oral ibandronate has been developed to overcome this problem.
Objective: To confirm the 1 year results and provide more extensive safety and tolerability information for once-monthly dosing by a 2 year analysis.
Methods: MOBILE, a randomised, phase III, non-inferiority study, compared the efficacy and safety of once-monthly ibandronate with daily ibandronate, which has previously been shown to reduce vertebral fracture risk in comparison with placebo.
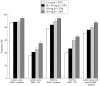
Results: 1609 postmenopausal women were randomised. Substantial increases in lumbar spine bone mineral density (BMD) were seen in all treatment arms: 5.0%, 5.3%, 5.6%, and 6.6% in the daily and once-monthly groups (50 + 50 mg, 100 mg, and 150 mg), respectively. It was confirmed that all once-monthly regimens were at least as effective as daily treatment, and in addition, 150 mg was found to be better (p<0.001). Substantial increases in proximal femur (total hip, femoral neck, trochanter) BMD were seen; 150 mg produced the most pronounced effect (p<0.05 versus daily treatment). Independent of the regimen, most participants (70.5-93.5%) achieved increases above baseline in lumbar spine or total hip BMD, or both. Pronounced decreases in the biochemical marker of bone resorption, sCTX, observed in all arms after 3 months, were maintained throughout. The 150 mg regimen consistently produced greater increases in BMD and sCTX suppression than the 100 mg and daily regimens. Ibandronate was well tolerated, with a similar incidence of adverse events across groups.
Conclusions: Once-monthly oral ibandronate is at least as effective and well tolerated as daily treatment. Once-monthly administration may be more convenient for patients and improve therapeutic adherence, thereby optimising outcomes.
Conflict of interest statement
References
-
- Melton L J, Chrischilles E A, Cooper C, Lane A W, Riggs B L. Perspective. How many women have osteoporosis? J Bone Miner Res 199271005–1010. - PubMed
-
- International Osteoporosis Foundation Osteoporosis in the European community: action plan. International Osteoporosis Foundation, 2003. Available from, http://www.osteofound.org/advocacy_policy/eu_policy_project/pdf/action_p... (accessed 12 January 2006)
-
- Melton L J. Adverse outcomes of osteoporotic fractures in the general population. J Bone Miner Res 2003181139–1141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources