Depletion of minichromosome maintenance protein 5 in the zebrafish retina causes cell-cycle defect and apoptosis
- PMID: 16339308
- PMCID: PMC1317923
- DOI: 10.1073/pnas.0506187102
Depletion of minichromosome maintenance protein 5 in the zebrafish retina causes cell-cycle defect and apoptosis
Abstract
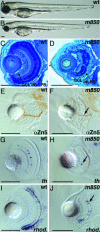
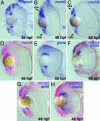
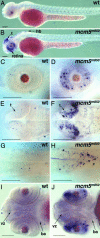
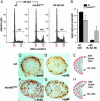
In multicellular organisms, the control of genome duplication and cell division must be tightly coordinated. Essential roles of the minichromosome maintenance (MCM) proteins for genome duplication have been well established. However, no genetic model has been available to address the function of MCM proteins in the context of vertebrate organogenesis. Here, we present positional cloning of a zebrafish mcm5 mutation and characterization of its retina phenotype. In the retina, mcm5 expression correlates closely with the pattern of cell proliferation. By the third day of development, mcm5 is down-regulated in differentiated cells but is maintained in regions containing retinal stem cells. We demonstrate that a gradual depletion of maternally derived MCM5 protein leads to a prolonged S phase, cell-cycle-exit failure, apoptosis, and reduction in cell number in mcm5(m850) mutant embryos. Interestingly, by the third day of development, increased apoptosis is detectable only in the retina, tectum, and hindbrain but not in other late-proliferating tissues, suggesting that different tissues may employ distinct cellular programs in responding to the depletion of MCM5.
Figures

References
-
- Donaldson, A. D. & Blow, J. J. (1999) Curr. Opin. Genet. Dev. 9, 62–68. - PubMed
-
- Diffley, J. F. X. & Labib, K. (2002) J. Cell. Sci. 115, 869–872. - PubMed
-
- Tye, B. K. (1999) Annu. Rev. Biochem. 68, 649–686. - PubMed
-
- Davey, M. J., Jeruzalmi, D., Kuriyan, J. & O'Donnell, M. (2002) Nat. Rev. Mol. Cell Biol. 3, 826–835. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

