An Ustilago maydis septin is required for filamentous growth in culture and for full symptom development on maize
- PMID: 16339722
- PMCID: PMC1317501
- DOI: 10.1128/EC.4.12.2044-2056.2005
An Ustilago maydis septin is required for filamentous growth in culture and for full symptom development on maize
Abstract
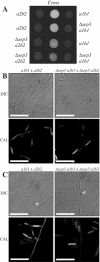
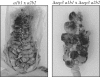
During maize infection, the fungal pathogen Ustilago maydis undergoes a dimorphic transition from budding, yeast-like cells to a filamentous dikaryon that proliferates in the host. This transition is regulated by mating and environmental signals. Septation is likely to be important in the growth of the infectious dikaryon because of the need to maintain specific cellular compartments during dikaryotic growth. Recently, we found that the transcript level for a septin gene was influenced by the conserved cyclic AMP (cAMP)/protein kinase A signaling pathway that participates in regulating dimorphism in U. maydis. In this study, we describe the detailed analysis of the function of this septin gene, designated sep3, in the growth, development, and pathogenesis of U. maydis. We show that sep3 is required for normal cellular morphology and the division of budding haploid cells. The gene is also required for lipid-induced filamentous growth in culture but not during the formation of mating filaments on agar medium or in planta. Strains with a deletion in sep3 have a reduction in symptom development in maize, with filamentous cells in planta displaying morphological defects. In addition, sep3 influences the differentiation of hyphae into teliospores and the germination of these teliospores to produce the meiotic haploid progeny that complete the disease life cycle. Finally, the deletion of sep3 was found to influence the multiple budding phenotype of a mutant with a defect in the regulatory subunit of protein kinase A. This result is consistent with a link between sep3 and the control of morphogenesis by cAMP signaling. Overall, this study highlights the importance of regulating septation and changes in morphology during phytopathogenesis.
Figures





References
-
- Andrews, D. L., J. D. Egan, M. E. Mayorga, and S. E. Gold. 2000. The Ustilago maydis ubc4 and ubc5 genes encode members of a MAP kinase cascade required for filamentous growth. Mol. Plant-Microbe Interact. 13:781-786. - PubMed
-
- Banuett, F., and I. Herskowitz. 1996. Discrete developmental stages during teliospore formation in the corn smut fungus, Ustilago maydis. Development 122:2965-2976. - PubMed
-
- Banuett, F., and I. Herskowitz. 1994. Identification of fuz7, a Ustilago maydis MEK/MAPKK homolog required for a-locus-dependent and -independent steps in the fungal life cycle. Genes Dev. 8:1367-1378. - PubMed
-
- Barrett, K. J., S. E. Gold, and J. W. Kronstad. 1993. Identification and complementation of a mutation to constitutive filamentous growth in Ustilago maydis. Mol. Plant-Microbe Interact. 6:274-283. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

