Flexural rigidity of individual microtubules measured by a buckling force with optical traps
- PMID: 16339879
- PMCID: PMC1367319
- DOI: 10.1529/biophysj.104.055483
Flexural rigidity of individual microtubules measured by a buckling force with optical traps
Abstract
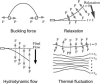
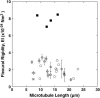
We used direct buckling force measurements with optical traps to determine the flexural rigidity of individual microtubules bound to polystyrene beads. To optimize the accuracy of the measurement, we used two optical traps and antibody-coated beads to manipulate each microtubule. We then applied a new analytical model assuming nonaxial buckling. Paclitaxel-stabilized microtubules were polymerized from purified tubulin, and the average microtubule rigidity was calculated as 2.0 x 10(-24) Nm2 using this novel microtubule buckling system. This value was not dependent on microtubule length. We also measured the rigidity of paclitaxel-free microtubules, and obtained the value of 7.9 x 10(-24) Nm2, which is nearly four times that measured for paclitaxel-stabilized microtubules.
Figures


References
-
- Howard, J. 2001. Mechanics of Motor Proteins and the Cytoskeleton. Sinauer Associates, Sunderland, MA.
-
- Mizushima-Sugano, J., T. Maeda, and T. Miki-Noumura. 1983. Flexural rigidity of singlet microtubules estimated from statistical analysis of their contour lengths and end-to-end distances. Biochim. Biophys. Acta. 755:257–262. - PubMed
-
- Kurachi, M., M. Hoshi, and H. Tashiro. 1995. Buckling of a single microtubule by optical trapping force: direct measurement of microtubule rigidity. Cell Motil. Cytoskeleton. 30:221–228. - PubMed
-
- Tran, P. T., S. F. Parsons, R. Sterba, Z. Wang, M. P. Sheetz, and E. D. Salmon. 1995. Direct measurement of microtubule flexural rigidity with the laser trap. Mol. Biol. Cell. 6:260a.
-
- Felgner, H., R. Frank, and M. Schliwa. 1996. Flexural rigidity of microtubules measured with the use of optical tweezers. J. Cell Sci. 109:509–516. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

