Temperature dependence of the volumetric parameters of drug binding to poly[d(A-T)].Poly[d(A-T)] and Poly(dA).Poly(dT)
- PMID: 16339884
- PMCID: PMC1367322
- DOI: 10.1529/biophysj.105.066258
Temperature dependence of the volumetric parameters of drug binding to poly[d(A-T)].Poly[d(A-T)] and Poly(dA).Poly(dT)
Abstract
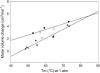
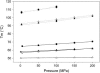
We report the temperature and salt dependence of the volume change (DeltaVb) associated with the binding of ethidium bromide and netropsin with poly(dA).poly(dT) and poly[d(A-T)].poly[d(A-T)]. The DeltaV(b) of binding of ethidium with poly(dA).poly(dT) was much more negative at temperatures approximately 70 degrees C than at 25 degrees C, whereas the difference is much smaller in the case of binding with poly[d(A-T)].poly[d(A-T)]. We also determined the volume change of DNA-drug interaction by comparing the volume change of melting of DNA duplex and DNA-drug complex. The DNA-drug complexes display helix-coil transition temperatures (Tm several degrees above those of the unbound polymers, e.g., the Tm of the netropsin complex with poly(dA)poly(dT) is 106 degrees C. The results for the binding of ethidium with poly[d(A-T)].poly[d(A-T)] were accurately described by scaled particle theory. However, this analysis did not yield results consistent with our data for ethidium binding with poly(dA).poly(dT). We hypothesize that heat-induced changes in conformation and hydration of this polymer are responsible for this behavior. The volumetric properties of poly(dA).poly(dT) become similar to those of poly[d(A-T)].poly[d(A-T)] at higher temperatures.
Figures



References
-
- Dervan, P. B. 2001. Molecular recognition of DNA by small molecules. Bioorg. Med. Chem. 9:2215–2235. - PubMed
-
- Haq, I., and J. Ladbury. 2000. Drug-DNA recognition: energetics and implications for design. J. Mol. Recognit. 13:188–197. - PubMed
-
- Marky, L. A., and R. B. Macgregor. 1990. Hydration of dA·dT polymers: role of water in the thermodynamics of ethidium and propidium intercalation. Biochemistry. 29:4805–4811. - PubMed
-
- Chalikian, T. V., G. E. Plum, A. P. Sarvazyan, and K. J. Breslauer. 1994. Influence of drug-binding on DNA hydration: acoustic and densimetric characterizations of netropsin binding to the poly(dAdT)·poly(dAdT) and poly(dA)·poly(dT) duplexes and the poly(dT)·poly(dA)·poly(dT) triplex at 25°C. Biochemistry. 33:8629–8640. - PubMed
-
- Marky, L. A., and D. W. Kupke. 1989. Probing the hydration of the minor groove of A.T synthetic DNA polymers by volume and heat changes. Biochemistry. 28:9982–9988. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

