Activation of naïve B lymphocytes via CD81, a pathogenetic mechanism for hepatitis C virus-associated B lymphocyte disorders
- PMID: 16339892
- PMCID: PMC1310512
- DOI: 10.1073/pnas.0509402102
Activation of naïve B lymphocytes via CD81, a pathogenetic mechanism for hepatitis C virus-associated B lymphocyte disorders
Abstract
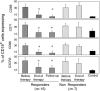
Infection with hepatitis C virus (HCV), a leading cause of chronic liver diseases, can associate with B lymphocyte proliferative disorders, such as mixed cryoglobulinemia and non-Hodgkin lymphoma. The major envelope protein of HCV (HCV-E2) binds, with high affinity CD81, a tetraspanin expressed on several cell types. Here, we show that engagement of CD81 on human B cells by a combination of HCV-E2 and an anti-CD81 mAb triggers the JNK pathway and leads to the preferential proliferation of the naïve (CD27-) B cell subset. In parallel, we have found that B lymphocytes from the great majority of chronic hepatitis C patients are activated and that naïve cells display a higher level of activation markers than memory (CD27+) B lymphocytes. Moreover, eradication of HCV infection by IFN therapy is associated with normalization of the activation-markers expression. We propose that CD81-mediated activation of B cells in vitro recapitulates the effects of HCV binding to B cell CD81 in vivo and that polyclonal proliferation of naïve B lymphocytes is a key initiating factor for the development of the HCV-associated B lymphocyte disorders.
Figures
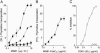


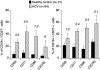
References
-
- Houghton, M. (1996) in Virology, eds. Fields, B. N., Knipe, D. M. & Howley, P. M. (Lippincott-Raven, Philadelphia), pp 1035-58.
-
- World Health Organization (1997) Weekly Epidemiol. Rec. 72, pp. 65-69.
-
- Hoofnagle, J. H. (1997) Hepatology 26, 15S-20S. - PubMed
-
- Agnello, V., Chung, R. T. & Kaplan, L. M. (1992) N. Engl. J. Med. 327, 1490-1495. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

