Solution structure of a multifunctional DNA- and protein-binding motif of human Werner syndrome protein
- PMID: 16339893
- PMCID: PMC1317980
- DOI: 10.1073/pnas.0509380102
Solution structure of a multifunctional DNA- and protein-binding motif of human Werner syndrome protein
Abstract
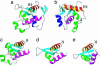
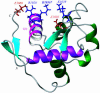
Werner syndrome (WS) is an autosomal recessive disease that results in premature aging. Mutations in the WS gene (WRN) result in a loss of expression of the WRN protein and predispose WS patients to accelerated aging. As a helicase and a nuclease, WRN is unique among the five human RecQ helicase family members and is capable of multiple functions involved in DNA replication, repair, recombination, and telomere maintenance. A 144-residue fragment of WRN was previously determined to be a multifunctional DNA- and protein-binding domain (DPBD) that interacts with structure-specific DNA and a variety of DNA-processing proteins. In addition, DPBD functions as a nucleolar targeting sequence of WRN. The solution structure of the DPBD, the first of a WRN fragment, has been solved by NMR. DPBD consists of a winged helix-like motif and an unstructured C-terminal region of approximately 20 aa. The putative DNA-binding surface of DPBD has been identified by using known structural and biochemical data. Based on the structural data and on the biochemical data, we suggest a surface on the DPBD for interacting with other proteins. In this structural model, a single winged helix domain binds to both DNA and other proteins. Furthermore, we propose that DPBD functions as a regulatory domain to regulate the enzymatic activity of WRN and to direct cellular localization of WRN through protein-protein interaction.
Figures




References
-
- Salk, D. (1982) Hum. Genet. 62, 1–5. - PubMed
-
- Poot, M., Hoehn, H., Runger, T. M. & Martin, G. M., (1992) Exp. Cell. Res. 202, 267–273. - PubMed
-
- Crabbe, L., Verdun, R. E., Haggblom, C. I. & Karlseder, J. (2004) Science 306, 1951–1953. - PubMed
-
- Yu, C. E., Oshima, J., Fu, Y. H., Wijsman, E. M., Hisama, F., Alisch, R., Matthews, S., Nakura, J., Miki, T., Ouais, S., et al. (1996) Science 272, 258–262. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

