Optical detection of rate-determining ion-modulated conformational changes of the ether-à-go-go K+ channel voltage sensor
- PMID: 16339906
- PMCID: PMC1317915
- DOI: 10.1073/pnas.0505766102
Optical detection of rate-determining ion-modulated conformational changes of the ether-à-go-go K+ channel voltage sensor
Abstract
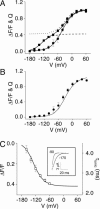
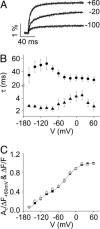
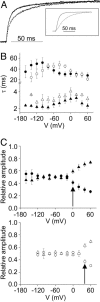
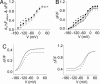
In voltage-dependent ether-à-go-go (eag) K+ channels, the process of activation is modulated by Mg2+ and other divalent cations, which bind to a site in the voltage sensor and slow channel opening. Previous analysis of eag ionic and gating currents indicated that Mg2+ has a much larger effect on ionic than gating current kinetics. From this, we hypothesized that ion binding modulates voltage sensor conformational changes that are poorly represented in gating current recordings. We have now tested this proposal by using a combined electrophysiological and optical approach. We find that a fluorescent probe attached near S4 in the voltage sensor reports on two phases of the activation process. One component of the optical signal corresponds to the main charge-moving conformational changes of the voltage sensor. This is the phase of activation that is well represented in gating current recordings. Another component of the optical signal reflects voltage sensor conformational changes that occur at more hyperpolarized potentials. These transitions, which are rate-determining for activation and highly modulated by Mg2+, have not been detected in gating current recordings. Our results demonstrate that the eag voltage sensor undergoes conformational changes that have gone undetected in electrical measurements. These transitions account for the time course of eag activation in the presence and absence of extracellular Mg2+.
Figures


References
-
- Warmke, J., Drysdale, R. & Ganetzky, B. (1991) Science 252, 1560-1562. - PubMed
-
- Sanguinetti, M. C., Jiang, C., Curran, M. E. & Keating, M. T. (1995) Cell 81, 299-307. - PubMed
-
- Curran, M. E., Splawski, I., Timothy, K. W., Vincent, G. M., Green, E. D. & Keating, M. T. (1995) Cell 80, 795-803. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

