Syk activation in dendritic cells is essential for airway hyperresponsiveness and inflammation
- PMID: 16339999
- PMCID: PMC2644204
- DOI: 10.1165/rcmb.2005-0298OC
Syk activation in dendritic cells is essential for airway hyperresponsiveness and inflammation
Abstract
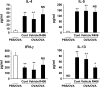
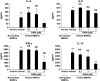
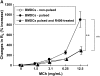
We evaluated the role of Syk, using an inhibitor, on allergen-induced airway hyperresponsiveness (AHR) and airway inflammation in a system shown to be B cell- and mast cell-independent. Sensitization of BALB/c mice with ovalbumin (OVA) and alum after three consecutive OVA challenges resulted in AHR to inhaled methacholine and airway inflammation. The Syk inhibitor R406 (30 mg/kg, administered orally, twice daily) prevented the development of AHR, increases in eosinophils and lymphocytes and IL-13 levels in bronchoalveolar lavage (BAL) fluid, and goblet cell metaplasia when administered after sensitization and before challenge with OVA. Levels of IL-4, IL-5, and IFN-gamma in BAL fluid and allergen-specific antibody levels in serum were not affected by treatment. Because many of these responses may be influenced by dendritic cell function, we investigated the effect of R406 on bone marrow-derived dendritic cell (BMDC) function. Co-culture of BMDC with immune complexes of OVA and IgG anti-OVA together with OVA-sensitized spleen mononuclear cells resulted in increases in IL-13 production. IL-13 production was inhibited if the BMDCs were pretreated with the Syk inhibitor. Intratracheal transfer of immune complex-pulsed BMDCs (but not nonpulsed BMDCs) to naive mice before airway allergen challenge induced the development of AHR and increases in BAL eosinophils and lymphocytes. All of these responses were inhibited if the transferred BMDCs were pretreated with R406. These results demonstrate that Syk inhibition prevents allergen-induced AHR and airway inflammation after systemic sensitization and challenge, at least in part through alteration of DC function.
Figures






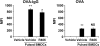
References
-
- Busse WW, Lemanske RF Jr. Asthma. N Engl J Med 2001;344:350–362. - PubMed
-
- Miyahara N, Swanson BJ, Takeda K, Taube C, Miyahara S, Kodama T, Dakhama A, Ott VL, Gelfand EW. Effector CD8+ T cells mediate inflammation and airway hyper-responsiveness. Nat Med 2004;10:865–869. - PubMed
-
- Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med 1992;326:298–304. - PubMed
-
- Kuipers H, Lambrecht BN. The interplay of dendritic cells, Th2 cells and regulatory T cells in asthma. Curr Opin Immunol 2004;16:702–708. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

