Therapeutics in renal disease: the road ahead for antiproliferative targets
- PMID: 16340240
- PMCID: PMC1440889
- DOI: 10.1159/000090138
Therapeutics in renal disease: the road ahead for antiproliferative targets
Abstract
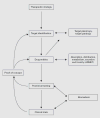
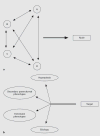
Discovery into the molecular basis of renal disease is occurring at an unprecedented rate. With the advent of the NIH Roadmap, there is a greater expectation of translating this knowledge into new treatments. Here, we review the therapeutic strategy to preserve renal function in proliferative renal diseases by directly inhibiting the mitogenic pathways within renal parenchymal cells that promote G0 to G1/S cell-cycle phase progression. Reductionist methodologies have identified several antiproliferative molecular targets, and promising preclinical testing of leading small-molecule drugs to modulate these targets has now led to landmark clinical trials. Yet, this advancement into targeted therapy highlights important differences between the therapeutic goals of molecular nephrology versus molecular oncology and, by extension, the poorly understood role of alternative target activity in drug efficacy. Systems research to clarify these issues should accelerate the development of this promising therapeutic strategy.
2006 S. Karger AG, Basel.
Figures

Similar articles
-
A comparative study of the effects of hemin and bilirubin on bilateral renal ischemia reperfusion injury.Nephron Exp Nephrol. 2006;103(1):e1-5. doi: 10.1159/000090113. Epub 2005 Dec 7. Nephron Exp Nephrol. 2006. PMID: 16340239
-
ETS-GS, a new antioxidant, ameliorates renal ischemia-reperfusion injury in a rodent model.J Surg Res. 2011 Nov;171(1):226-33. doi: 10.1016/j.jss.2010.01.039. Epub 2010 Feb 23. J Surg Res. 2011. PMID: 20451924
-
Influence of heme oxygenase-1 on microcirculation after kidney transplantation.J Surg Res. 2008 Aug;148(2):126-35. doi: 10.1016/j.jss.2007.10.007. Epub 2007 Nov 26. J Surg Res. 2008. PMID: 18456280
-
CDK/GSK-3 inhibitors as therapeutic agents for parenchymal renal diseases.Kidney Int. 2008 Mar;73(6):684-90. doi: 10.1038/sj.ki.5002731. Epub 2007 Dec 19. Kidney Int. 2008. PMID: 18094678 Review.
-
Renal Pre-Competitive Consortium (RPC2): discovering therapeutic targets together.Drug Discov Today. 2018 Oct;23(10):1695-1699. doi: 10.1016/j.drudis.2018.05.021. Epub 2018 May 18. Drug Discov Today. 2018. PMID: 29778696 Review.
Cited by
-
The cell cycle and acute kidney injury.Kidney Int. 2009 Sep;76(6):604-13. doi: 10.1038/ki.2009.224. Epub 2009 Jun 17. Kidney Int. 2009. PMID: 19536080 Free PMC article. Review.
-
CDK4/6 inhibition induces epithelial cell cycle arrest and ameliorates acute kidney injury.Am J Physiol Renal Physiol. 2014 Feb 15;306(4):F379-88. doi: 10.1152/ajprenal.00475.2013. Epub 2013 Dec 11. Am J Physiol Renal Physiol. 2014. PMID: 24338822 Free PMC article.
References
-
- Shankland SJ. Cell-cycle control and renal disease. Kidney Int. 1997;52:294–308. - PubMed
-
- Zerhouni E. Medicine. The NIH Roadmap Science. 2003;302:63–72. - PubMed
-
- Coppo R, Amore A. New perspectives in treatment of glomerulonephritis. Pediatr Nephrol. 2004;19:256–265. - PubMed
-
- Javaid B, Quigg R. Treatment of glomerulonephritis: will we ever have options other than steroids and cytotoxics? Kidney Int. 2005;67:1692–1703. - PubMed
-
- Pabst R, Sterzel RB. Cell renewal of glomerular cell types in normal rats. an autoradiographic analysis. Kidney Int. 1983;24:626–631. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

