Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients
- PMID: 16341266
- PMCID: PMC1307558
- DOI: 10.1172/JCI21759
Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients
Abstract
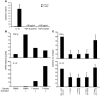
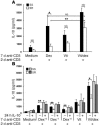
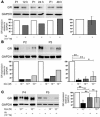
We previously reported that human CD4+ Tregs secrete high levels of IL-10 when stimulated in the presence of dexamethasone and calcitriol (vitamin D3). We now show that following stimulation by allergen, IL-10-secreting Tregs inhibit cytokine secretion by allergen-specific Th2 cells in an IL-10-dependent manner. A proportion of patients with severe asthma fail to demonstrate clinical improvement upon glucocorticoid therapy, and their asthma is characterized as glucocorticoid resistant (SR, abbreviation derived from "steroid resistant"). Dexamethasone does not enhance secretion of IL-10 by their CD4+ T cells. Addition of vitamin D3 with dexamethasone to cultures of SR CD4+ T cells enhanced IL-10 synthesis to levels observed in cells from glucocorticoid-sensitive patients cultured with dexamethasone alone. Furthermore, pretreatment with IL-10 fully restored IL-10 synthesis in these cells in response to dexamethasone. Vitamin D3 significantly overcame the inhibition of glucocorticoid-receptor expression by dexamethasone while IL-10 upregulated glucocorticoid-receptor expression by CD4+ T cells, suggesting potential mechanisms whereby these treatments may overcome poor glucocorticoid responsiveness. We show here that administration of vitamin D3 to healthy individuals and SR asthmatic patients enhanced subsequent responsiveness to dexamethasone for induction of IL-10. This strongly suggests that vitamin D3 could potentially increase the therapeutic response to glucocorticoids in SR patients.
Figures




References
-
- Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 1999;17:255–281. - PubMed
-
- Umland SP, Schleimer RP, Johnston SL. Review of the molecular and cellular mechanisms of action of glucocorticoids for use in asthma. Pulm. Pharmacol. Ther. 2002;15:35–50. - PubMed
-
- Richards DF, Fernandez M, Caulfield J, Hawrylowicz CM. Glucocorticoids drive human CD8(+) T cell differentiation towards a phenotype with high IL-10 and reduced IL-4, IL-5 and IL-13 production. Eur. J. Immunol. 2000;30:2344–2354. - PubMed
-
- John M, et al. Inhaled corticosteroids increase interleukin-10 but reduce macrophage inflammatory protein-1α, granulocyte-macrophage colony stimulating factor and interferon-γ release from alveolar macrophages in asthma. Am. J. Respir. Crit. Care Med. 1998;157:256–262. - PubMed
-
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

