Molecular mimicry: sensitization of Lewis rats with Campylobacter jejuni lipopolysaccharides induces formation of antibody toward GD3 ganglioside
- PMID: 16342208
- PMCID: PMC2762320
- DOI: 10.1002/jnr.20717
Molecular mimicry: sensitization of Lewis rats with Campylobacter jejuni lipopolysaccharides induces formation of antibody toward GD3 ganglioside
Abstract
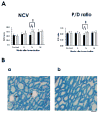
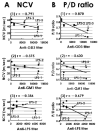
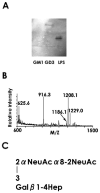
Recently we have reported cases of demyelinating inflammatory neuropathy showing elevated titers of anti-GD3 antibodies, which occurs rarely in Guillain-Barré syndrome. To examine the correlation between the anti-GD3 antibody titer and Campylobacter jejuni infection, we sensitized female Lewis rats with lipopolysaccharides (LPSs) from serotype HS19 of C. jejuni and examined changes in nerve conduction velocity and nerve conduction block (P/D ratio). After 16 weeks of sensitization, animals revealed decreases of nerve conduction velocity and conduction block (P/D ratio) and high titer of anti-GD3 antibodies. These anti-GD3 antibodies also blocked transmission in neuromuscular junctions of spinal cord-muscle cells cocultures. The GD3 epitope was verified to be located on the Schwann cell surface and nodes of Ranvier in rat sciatic nerve. To determine the target epitope for GD3 antibodies in causing nerve dysfunction, the LPS fraction containing the GD3 epitope was purified from the total LPS by using an anti-GD3 monoclonal antibody-immobilized affinity column. Subsequently, chemical analysis of the oligosaccharide portion was performed and confirmed the presence of a GD3-like epitope as having the following tetrasaccharide structure: NeuAcalpha2-8NeuAc2-3Galbeta1-4Hep. Our data thus support the possibility of a contribution of GD3 mimicry as a potential pathogenic mechanism of peripheral nerve dysfunction.
Copyright 2005 Wiley-Liss, Inc.
Figures
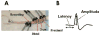
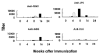


Similar articles
-
Monoclonal antibodies raised against Guillain-Barré syndrome-associated Campylobacter jejuni lipopolysaccharides react with neuronal gangliosides and paralyze muscle-nerve preparations.J Clin Invest. 1999 Sep;104(6):697-708. doi: 10.1172/JCI6837. J Clin Invest. 1999. PMID: 10491405 Free PMC article.
-
Development of a novel therapy for Lipo-oligosaccharide-induced experimental neuritis: use of peptide glycomimics.J Neurochem. 2010 Apr;113(2):351-62. doi: 10.1111/j.1471-4159.2010.06627.x. Epub 2010 Feb 1. J Neurochem. 2010. PMID: 20132479 Free PMC article.
-
Tolerance to self gangliosides is the major factor restricting the antibody response to lipopolysaccharide core oligosaccharides in Campylobacter jejuni strains associated with Guillain-Barré syndrome.Infect Immun. 2002 Sep;70(9):5008-18. doi: 10.1128/IAI.70.9.5008-5018.2002. Infect Immun. 2002. PMID: 12183547 Free PMC article.
-
[Pathogenesis of Guillain-Barré syndrome and Fisher's syndrome: molecular mimicry between antecedent infectious agents and components of nerve tissues].Rinsho Shinkeigaku. 1995 Dec;35(12):1373-5. Rinsho Shinkeigaku. 1995. PMID: 8752400 Review. Japanese.
-
Molecular mimicry between gangliosides and lipopolysaccharides of Campylobacter jejuni isolated from patients with Guillain-Barré syndrome and Miller Fisher syndrome.J Infect Dis. 1997 Dec;176 Suppl 2:S150-3. doi: 10.1086/513800. J Infect Dis. 1997. PMID: 9396700 Review.
Cited by
-
Ganglioside molecular mimicry and its pathological roles in Guillain-Barré syndrome and related diseases.Infect Immun. 2006 Dec;74(12):6517-27. doi: 10.1128/IAI.00967-06. Epub 2006 Sep 11. Infect Immun. 2006. PMID: 16966405 Free PMC article. Review. No abstract available.
-
Topology and patch-clamp analysis of the sodium channel in relationship to the anti-lipid a antibody in campylobacteriosis.J Neurosci Res. 2008 Nov 15;86(15):3359-74. doi: 10.1002/jnr.21781. J Neurosci Res. 2008. PMID: 18627035 Free PMC article.
-
Novel anti-idiotype antibody therapy for lipooligosaccharide-induced experimental autoimmune neuritis: use relevant to Guillain-Barré syndrome.J Neurosci Res. 2010 Jun;88(8):1651-63. doi: 10.1002/jnr.22330. J Neurosci Res. 2010. PMID: 20077429 Free PMC article.
-
Miller Fisher Syndrome Associated With COVID-19: A History of Molecular Mimicry and an Up-to-Date Review of the Literature.Cureus. 2023 Aug 8;15(8):e43111. doi: 10.7759/cureus.43111. eCollection 2023 Aug. Cureus. 2023. PMID: 37692684 Free PMC article. Review.
-
Antiganglioside antibodies and their pathophysiological effects on Guillain-Barré syndrome and related disorders--a review.Glycobiology. 2009 Jul;19(7):676-92. doi: 10.1093/glycob/cwp027. Epub 2009 Feb 24. Glycobiology. 2009. PMID: 19240270 Free PMC article. Review.
References
-
- Andersen H, Nielsen JF, Nielsen KN. Inability of insulin to maintain normal nerve function during high-frequency stimulation in diabetic rat tail nerves. Muscle Nerve. 1994;17:80–84. - PubMed
-
- Ang CW, Jacobs BC, Laman JD. The Guillain-Barré syndrome: a true case of molecular mimicry. Trend Immunol. 2004;25:61–66. - PubMed
-
- Arasaki K, Kusunoki S, Kudo N, Tamaki M. The pattern of anti-ganglioside antibody reactivities producing myelinated nerve conduction block in vitro. J Neurol Sci. 1998;161:163–168. - PubMed
-
- Ariga T, Yu RK. Antiglycolipid antibodies in Guillain-Barré syndrome and related diseases: review of clinical features and antibody specificties. J Neurosci Res. 2005;80:1–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

