A missense mutation in the bovine SLC35A3 gene, encoding a UDP-N-acetylglucosamine transporter, causes complex vertebral malformation
- PMID: 16344554
- PMCID: PMC1356133
- DOI: 10.1101/gr.3690506
A missense mutation in the bovine SLC35A3 gene, encoding a UDP-N-acetylglucosamine transporter, causes complex vertebral malformation
Abstract
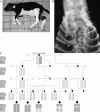
The extensive use of a limited number of elite bulls in cattle breeding can lead to rapid spread of recessively inherited disorders. A recent example is the globally distributed syndrome Complex Vertebral Malformation (CVM), which is characterized by misshapen and fused vertebrae around the cervico-thoracic junction. Here, we show that CVM is caused by a mutation in the Golgi-resident nucleotide-sugar transporter encoded by SLC35A3. Thus, the disease showed complete cosegregation with the mutation in a homozygous state, and proteome patterns indicated abnormal protein glycosylation in tissues of affected animals. In addition, a yeast mutant that is deficient in the transport of UDP-N-acetylglucosamine into its Golgi lumen can be rescued by the wild-type SLC35A3 gene, but not by the mutated gene. These results provide the first demonstration of a genetic disorder associated with a defective SLC35A3 gene, and reveal a new mechanism for malformation of the vertebral column caused by abnormal nucleotide-sugar transport into the Golgi apparatus.
Figures


References
-
- Abeijon, C., Mandon, E.C., and Hirschberg, C.B. 1997. Transporters of nucleotide sugars, nucleotide sulfate and ATP in the Golgi apparatus. Trends Biochem. Sci. 22 203-207. - PubMed
-
- Agerholm, J.S., Bendixen, C., Andersen, O., and Arnbjerg, J. 2001. Complex vertebral malformation in holstein calves. J. Vet. Diagn. Invest. 13 283-289. - PubMed
-
- Agerholm, J.S., Bendixen, C., Arnbjerg, J., and Andersen, O. 2004. Morphological variation of “complex vertebral malformation” in Holstein calves. J. Vet. Diagn. Invest. 16 548-553. - PubMed
Web site references
-
- http://prowl.rockefeller.edu/profound_bin/WebProFound.exe; ProFound tool for searching protein sequence databases.
-
- http://www.phrap.org; PHRAP assembly engine.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
