Microbial consumption of atmospheric isoprene in a temperate forest soil
- PMID: 16349477
- PMCID: PMC124689
- DOI: 10.1128/AEM.64.1.172-177.1998
Microbial consumption of atmospheric isoprene in a temperate forest soil
Abstract
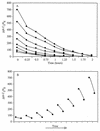
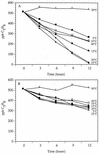
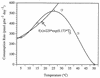
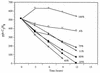
Isoprene (2-methyl-1,3 butadiene) is a low-molecular-weight hydrocarbon emitted in large quantities to the atmosphere by vegetation and plays a large role in regulating atmospheric chemistry. Until now, the atmosphere has been considered the only significant sink for isoprene. However, in this study we performed both in situ and in vitro experiments with soil from a temperate forest near Ithaca, N.Y., that indicate that the soil provides a sink for atmospheric isoprene and that the consumption of isoprene is carried out by microorganisms. Consumption occurred rapidly in field chambers (672.60 +/- 30.12 to 2,718.36 +/- 86.40 pmol gdw day) (gdw is grams [dry weight] of soil; values are means +/- standard deviations). Subsequent laboratory experiments confirmed that isoprene loss was due to biological processes: consumption was stopped by autoclaving the soil; consumption rates increased with repeated exposure to isoprene; and consumption showed a temperature response consistent with biological activity (with an optimum temperature of 30 degrees C). Isoprene consumption was diminished under low oxygen conditions (120 +/- 7.44 versus 528.36 +/- 7.68 pmol gdw day under ambient O(2) concentrations) and showed a strong relationship with soil moisture. Isoprene-degrading microorganisms were isolated from the site, and abundance was calculated as 5.8 x 10 +/- 3.2 x 10 cells gdw. Our results indicate that soil may provide a significant biological sink for atmospheric isoprene.
Figures

References
-
- Ashton F M. Persistence and biodegradation of herbicides. In: Matsumura F M, Krishna Murti C R, editors. Biodegradation of pesticides. New York, N.Y: Plenum Press; 1982.
-
- Atlas R M, Bartha R. Microbial ecology. Redwood City, Calif: The Benjamin/Cummings Publishing Company; 1993.
-
- Audus L J. Herbicide behavior in the soil. In: Audus L J, editor. The physiology and biochemistry of herbicides. New York, N.Y: Academic Press; 1964.
-
- Baldocci D, Guenther A, Harley P, Klinger L, Zimmerman P, Lamb B, Westberg H. The fluxes and air chemistry of isoprene above a deciduous hardwood forest. Philos Trans R Soc London. 1995;350:279–296. - PubMed
-
- Bender M, Conrad R. Kinetics of methane oxidation in oxic soils. Chemosphere. 1993;26:687–696.
LinkOut - more resources
Full Text Sources

