Purification and Properties of a Polyester Polyurethane-Degrading Enzyme from Comamonas acidovorans TB-35
- PMID: 16349494
- PMCID: PMC124672
- DOI: 10.1128/AEM.64.1.62-67.1998
Purification and Properties of a Polyester Polyurethane-Degrading Enzyme from Comamonas acidovorans TB-35
Abstract
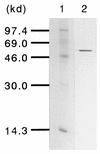
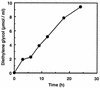
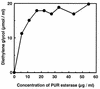
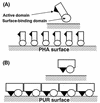
A polyester polyurethane (PUR)-degrading enzyme, PUR esterase, derived from Comamonas acidovorans TB-35, a bacterium that utilizes polyester PUR as the sole carbon source, was purified until it showed a single band in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). This enzyme was bound to the cell surface and was extracted by addition of 0.2% N,N-bis(3-d-gluconamidopropyl)deoxycholamide (deoxy-BIGCHAP). The results of gel filtration and SDS-PAGE showed that the PUR esterase was a monomer with a molecular mass of about 62,000 Da. This enzyme, which is a kind of esterase, degraded solid polyester PUR, with diethylene glycol and adipic acid released as the degradation products. The optimum pH for this enzyme was 6.5, and the optimum temperature was 45 degrees C. PUR degradation by the PUR esterase was strongly inhibited by the addition of 0.04% deoxy-BIGCHAP. On the other hand, deoxy-BIGCHAP did not inhibit the activity when p-nitrophenyl acetate, a water-soluble compound, was used as a substrate. These observations indicated that this enzyme degrades PUR in a two-step reaction: hydrophobic adsorption to the PUR surface and hydrolysis of the ester bond of PUR.
Figures



References
-
- Behrends A, Klingbeil B, Jendrossek D. Poly(3-hydroxybutyrate) depolymerases bind to their substrate by a C-terminal located substrate binding site. FEMS Microbiol Lett. 1996;143:191–194. - PubMed
-
- Crabbe J R, Campbell J R, Thompson L, Walz S L, Schultz W W. Biodegradation of a colloidal ester-based polyurethane by soil fungi. Int Biodeterior Biodegrad. 1994;33:103–113.
-
- Fukui T, Narikawa T, Miwa K, Shirakura Y, Saito T, Tomita K. Effect of limited tryptic modification of a bacterial poly(3-hydroxybutyrate) depolymerase on its catalytic activity. Biochim Biophys Acta. 1988;952:164–171. - PubMed
-
- Hansen C K. Fibronectin type III-like sequences and a new domain type in prokaryotic depolymerases with insoluble substrates. FEBS Lett. 1992;305:91–96. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

