Oxidative cyclizations in a nonpolar solvent using molecular oxygen and studies on the stereochemistry of oxypalladation
- PMID: 16351107
- PMCID: PMC2585987
- DOI: 10.1021/ja055534k
Oxidative cyclizations in a nonpolar solvent using molecular oxygen and studies on the stereochemistry of oxypalladation
Abstract
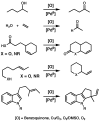
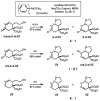
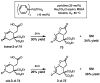
Oxidative cyclizations of a variety of heteroatom nucleophiles onto unactivated olefins are catalyzed by palladium(II) and pyridine in the presence of molecular oxygen as the sole stoichiometric oxidant in a nonpolar solvent (toluene). Reactivity studies of a number of N-ligated palladium complexes show that chelating ligands slow the reaction. Nearly identical conditions are applicable to five different types of nucleophiles: phenols, primary alcohols, carboxylic acids, a vinylogous acid, and amides. Electron-rich phenols are excellent substrates, and multiple olefin substitution patterns are tolerated. Primary alcohols undergo oxidative cyclization without significant oxidation to the aldehyde, a fact that illustrates the range of reactivity available from various Pd(II) salts under differing conditions. Alcohols can form both fused and spirocyclic ring systems, depending on the position of the olefin relative to the tethered alcohol; the same is true of the acid derivatives. The racemic conditions served as a platform for the development of an enantioselective reaction. Experiments with stereospecifically deuterated primary alcohol substrates rule out a "Wacker-type" mechanism involving anti oxypalladation and suggest that the reaction proceeds by syn oxypalladation for both mono- and bidentate ligands. In contrast, cyclizations of deuterium-labeled carboxylic acid substrates undergo anti oxypalladation.
Figures
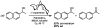



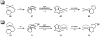
References
-
-
Johnson RA, Sharpless KB. In: Catalytic Asymmetric Synthesis. Ojima I, editor. Wiley & Sons, Inc; New York: 2000. pp. 231–280.Katsuki T. In: Catalytic Asymmetric Synthesis. Ojima I, editor. Wiley & Sons, Inc; New York: 2000. pp. 287–325.Jacobsen EN. In: Comprehensive Asymmetric 21 Catalysis. Jaobsen EN, Pfaltz A, Yamamoto H, editors. Vol. 2. Springer; Berlin: 1999. pp. 607–618.For a recent review of advances in transition metal catalyzed oxidation, see: Punniyamurthy T, Velusamy S, Iqbal J. Chem Rev. 2005;105:2329–2364.
-
-
- Tsuji J. Palladium Reagents and Catalysis. John Wiley & Sons, Ltd; Chichester, UK; Hoboken, NJ: 2004.
- Negishi E, editor. Handbook of Organopalladium Chemistry for Organic Synthesis. Wiley & Sons, Inc; New York: 2002.
- Tietze LF, Ila H, Bell HP. Chem Rev. 2004;104:3453–3516. - PubMed
-
-
For reviews, see: Stoltz BM. Chem Lett. 2004;33:362–367.Stahl SS. Angew Chem, Int Ed. 2004;43:3400–3420.Sigman MS, Schultz MJ. Org Biomol Chem. 2004;2:2551–2554.For a recent review of Pd-catalyzed alcohol oxidation, see: Muzart J. Tetraherdon. 2003;59:5789–5816.Trost BM. Acc Chem Res. 1990;23:34–42.Hedgedus LS. Tetrahedron. 1984;40:2415–2434.Hosokawa T, Murahashi SI. Acc Chem Res. 1990;23:49–54.Semmelhack MF, Kim C, Zhang N, Bodurow C, Sanner M, Dobler W, Meier M. Pure Appl Chem. 1990;23:2035–2040.Tsuji J. Palladium Reagents and Catalysts. John Wiley & Sons, Ltd; Chichester, UK: 1995. pp. 125–527.Zeni G, Larock RC. Chem Rev. 2004;104:2285–2309.Wolfe JP, Thomas JS. Curr Org Chem. 2005;9:625–655.
-
-
- Blackburn TF, Schwartz J. J Chem Soc, Chem Commun. 1977:157–158.
-
-
For examples of phenol cyclizations, see: Larock RC, Wei L, Hightower T. Synlett. 1998:522–524.For examples of alcohol cyclizations, see: Rönn M, Bäckvall J-E, Andersson PG. Tetrahedron Lett. 1995;36:7749–7752.For examples of acid cyclizations, see: Larock RC, Hightower TR. J Org Chem. 1993;58:5298–5300.For examples of tosylamide cyclizations, see: Larock RC, Hightower TR, Hasvold LA, Peterson KP. J Org Chem. 1996;61:3584–3585. and references therein.For examples of primary and secondary alcohol oxidation to aldehydes and ketones, see: Peterson KP, Larock RC. J Org Chem. 1998;63:3185–3189.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

