Evolution of ciliary patterns in the Oligotrichida (Ciliophora, Spirotricha) and its taxonomic implications
- PMID: 16351935
- PMCID: PMC2848327
- DOI: 10.1016/j.zool.2004.02.003
Evolution of ciliary patterns in the Oligotrichida (Ciliophora, Spirotricha) and its taxonomic implications
Abstract
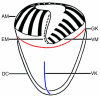
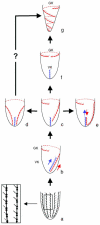
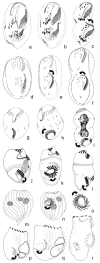
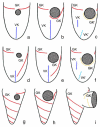
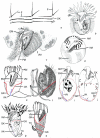
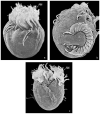
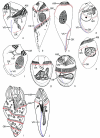
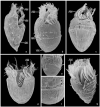
Although the somatic ciliature of the Oligotrichida typically comprises only a girdle and ventral kinety, a considerable diversity of ciliary patterns occurs. The four main girdle kinety patterns are identically found in tailed and tail-less species. The contractile tail has a complicated and unique ultrastructure and is potentially useful for the cell's movement and/or stabilization during feeding. Accordingly, I assume that this structure has evolved only once, namely, in the Tontoniidae nov. fam., and that the different girdle kinety patterns developed convergently in the tailed and tail-less taxa. Further distinct features suggest the establishment of the families Cyrtostrombidiidae nov. fam. (with cyrtos-like pharyngeal fibres and lack of ventral membranelles and endoral) and Pelagostrombidiidae nov. fam. (with neoformation organelle). An attempt is made to reconstruct the evolution of the kinety patterns based on morphologic, ontogenetic, and ultrastructural data. Some genera of tail-less Oligotrichida base on differences in the ciliary pattern; Omegastrombidium nov. gen. is erected for a further girdle kinety pattern. Likewise, the tailed genus Tontonia is split, resulting in two new genera, viz., Pseudotontonia nov. gen. and Spirotontonia nov. gen. Furthermore, the genus Spirostrombidium is split due to the different origin of the parallel course of girdle and ventral kinety, and Parallelostrombidium nov. gen. is established. However, the genus Thigmostrombidium is rejected because its enlarged thigmotactic membranelles are interpreted as an adaptation to the benthic lifestyle, which occurred several times within different girdle kinety patterns.
Figures
References
-
- Aescht E. Catalogue of the generic names of ciliates (Protozoa, Ciliophora) Denisia. 2001;1
-
- Agatha S. Morphology and ontogenesis of Novistrombidium apsheronicum nov. comb. and Strombidium arenicola (Protozoa: Ciliophora): a comparative light microscopical and SEM study. Eur. J. Protistol. 2003;39:245–266.
-
- Agatha S, Riedel-Lorjé JC. Morphology, infraciliature, and ecology of halteriids and strombidiids (Ciliophora, Oligotrichea) from coastal brackish water basins. Arch. Protistenkd. 1997;148:445–459.
Grants and funding
LinkOut - more resources
Full Text Sources

