Identification of a novel pathway essential for the immediate-early, interferon-independent antiviral response to enveloped virions
- PMID: 16352547
- PMCID: PMC1317555
- DOI: 10.1128/JVI.80.1.226-235.2006
Identification of a novel pathway essential for the immediate-early, interferon-independent antiviral response to enveloped virions
Abstract
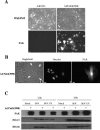
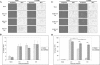
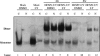
Viral infection elicits the activation of numerous cellular signal transduction pathways, leading to the induction of both innate and adaptive immunity. Previously we showed that entry of virion particles from a diverse array of enveloped virus families was capable of eliciting an interferon regulatory factor 3 (IRF-3)-mediated antiviral state in human fibroblasts in the absence of interferon production. Here we show that extracellular regulated kinase 1/2, p38 mitogen-activated protein kinase, and Jun N-terminal kinase/stress-activated protein kinase activities are not required for antiviral state induction. In contrast, treatment of cells with LY294002, an inhibitor of the phosphoinositide 3-kinase (PI3 kinase) family, prevents the induction of interferon-stimulated gene 56 (ISG56) and an antiviral response upon entry of virus particles. However, the prototypic class I p85/p110 PI3 kinase and its downstream effector Akt/PKB are dispensable for ISG and antiviral state induction. Furthermore, DNA-PK and PAK1, LY294002-sensitive members of the PI3 kinase family shown previously to be involved in IRF-3 activation, are also dispensable for ISG and antiviral state induction. The LY294002 inhibitor fails to prevent IRF-3 homodimerization or nuclear translocation upon virus particle entry. Together, these data suggest that virus entry triggers an innate antiviral response that requires the activity of a novel PI3 kinase family member.
Figures




References
-
- Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413:732-738. - PubMed
-
- Balachandran, S., E. Thomas, and G. N. Barber. 2004. A FADD-dependent innate immune mechanism in mammalian cells. Nature 432:401-405. - PubMed
-
- Bennett, B. L., D. T. Sasaki, B. W. Murray, E. C. O'Leary, S. T. Sakata, W. Xu, J. C. Leisten, A. Motiwala, S. Pierce, Y. Satoh, S. S. Bhagwat, A. M. Manning, and D. W. Anderson. 2001. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA 98:13681-13686. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

