Expanded tropism and altered activation of a retroviral glycoprotein resistant to an entry inhibitor peptide
- PMID: 16352560
- PMCID: PMC1317511
- DOI: 10.1128/JVI.80.1.353-359.2006
Expanded tropism and altered activation of a retroviral glycoprotein resistant to an entry inhibitor peptide
Abstract
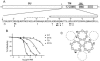
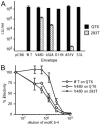
The envelope of class I viruses can be a target for potent viral inhibitors, such as the human immunodeficiency virus type 1 (HIV-1) inhibitor enfuvirtide, which are derived from the C-terminal heptad repeat (HR2) of the transmembrane (TM) subunit. Resistance to an HR2-based peptide inhibitor of a model retrovirus, subgroup A of the Avian Sarcoma and Leukosis Virus genus (ASLV-A), was studied by examining mutants derived by viral passage in the presence of inhibitor. Variants with reduced sensitivity to inhibitor were readily selected in vitro. Sensitivity determinants were identified for 13 different isolates, all of which mapped to the TM subunit. These determinants were identified in two regions: (i) the N-terminal heptad repeat (HR1) and (ii) the N-terminal segment of TM, between the subunit cleavage site and the fusion peptide. The latter class of mutants identified a region outside of the predicted HR2-binding site that can significantly alter sensitivity to inhibitor. A subset of the HR1 mutants displayed the unanticipated ability to infect nonavian cells. This expanded tropism was associated with increased efficiency of envelope triggering by soluble receptor at low temperatures, as measured by protease sensitivity of the surface subunit (SU) of envelope. In addition, expanded tropism was linked for the most readily triggered mutants with increased sensitivity to neutralization by SU-specific antiserum. These observations depict a class of HR2 peptide-selected mutations with a reduced activation threshold, thereby allowing the utilization of alternative receptors for viral entry.
Figures

References
-
- Balliet, J. W. 1998. Early events in subgroup A avian sarcoma and leukosis virus entry. Ph.D. thesis. University of Pennsylvania, Philadelphia, Pa.
-
- Bates, P., J. A. T. Young, and H. E. Varmus. 1993. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell 74:1043-1051. - PubMed
-
- Bosch, B. J., B. E. E. Martina, R. van der Zee, J. Lepault, B. J. Haijema, C. Versluis, A. J. R. Heck, R. de Groot, A. D. M. E. Osterhaus, and P. J. M. Rottier. 2004. Severe acute respiratory syndrome coronavirus (SARS-CoV) infection inhibition using spike protein heptad repeat-derived peptides. Proc. Natl. Acad. Sci. USA 101:8455-8460. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

