The herpes simplex virus type 1 vhs-UL41 gene secures viral replication by temporarily evading apoptotic cellular response to infection: Vhs-UL41 activity might require interactions with elements of cellular mRNA degradation machinery
- PMID: 16352574
- PMCID: PMC1317524
- DOI: 10.1128/JVI.80.1.505-513.2006
The herpes simplex virus type 1 vhs-UL41 gene secures viral replication by temporarily evading apoptotic cellular response to infection: Vhs-UL41 activity might require interactions with elements of cellular mRNA degradation machinery
Abstract
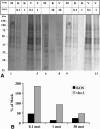
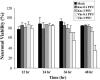
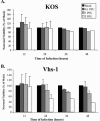
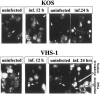
We have previously shown that herpes simplex virus type 1 (HSV-1) infection is associated with early destabilization/degradation of infected cell mRNAs and consequent shutoff of host protein synthesis by the activity of the virion-associated host shutoff (vhs) UL41 protein. Wild-type (wt) virus destabilized/degraded the housekeeping beta-actin and alpha-tubulin mRNAs as well host stress functions, like the heat shock 70 protein induced postinfection. vhs mutants did not degrade the mRNAs. Elaborate studies by others have been concerned with the mode of mRNA degradation and the mRNAs affected. We now describe vhs activity in primary cultures of mouse cerebellar granule neurons (CGNs). Specifically, (i) upon infection in the presence of actinomycin D to test activity of input viral particles, there was a generalized inhibition of protein synthesis, which depended on the input multiplicity of infection (MOI). (ii) Low-MOI infection with vhs-1 mutant virus was associated with increased synthesis of all apparent proteins. Higher MOIs caused some shutoff, albeit significantly lower than that of wt virus. This pattern could reflect an interaction(s) of vhs-1 protein with host machinery involved in cellular mRNA destabilization/degradation, sequestering this activity. (iii) wt virus infection was associated with cell survival, at least for a while, whereas mutant virus induced apoptotic cell death at earlier times. (iv) wt virus replicated well in the CGNs, whereas there was no apparent replication of the vhs-1 mutant virus. (v) The vhs-1 mutant could serve as helper virus for composite amplicon vectors carrying marker genes and the human p53 gene. Ongoing studies test the use of vhs-1-based composite oncolytic vectors towards cancer gene therapy.
Figures


References
-
- Aubert, M., and J. A. Blaho. 2001. Modulation of apoptosis during herpes simplex virus infection in human cells. Microbes Infect. 3:859-866. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

