Augmenting neurotransmitter release by enhancing the apparent Ca2+ affinity of synaptotagmin 1
- PMID: 16352718
- PMCID: PMC1311909
- DOI: 10.1073/pnas.0509153102
Augmenting neurotransmitter release by enhancing the apparent Ca2+ affinity of synaptotagmin 1
Abstract
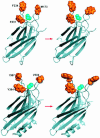
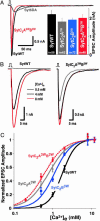
Synaptotagmin 1 likely acts as a Ca2+ sensor in neurotransmitter release by Ca2+-binding to its two C2 domains. This notion was strongly supported by the observation that a mutation in the C2A domain causes parallel decreases in the apparent Ca2+ affinity of synaptotagmin 1 and in the Ca2+ sensitivity of release. However, this study was based on a single loss-of-function mutation. We now show that tryptophan substitutions in the synaptotagmin 1 C2 domains act as gain-of-function mutations to increase the apparent Ca2+ affinity of synaptotagmin 1. The same substitutions, when introduced into synaptotagmin 1 expressed in neurons, enhance the Ca2+ sensitivity of release. Mutations in the two C2 domains lead to comparable and additive effects in release. Our results thus show that the apparent Ca2+ sensitivity of release is dictated by the apparent Ca2+ affinity of synaptotagmin 1 in both directions, and that Ca2+ binding to both C2 domains contributes to Ca2+ triggering of release.
Figures



References
-
- Chapman, E. R. (2002) Nat. Rev. Mol. Cell Biol. 3, 498-508. - PubMed
-
- Südhof, T. C. (2004) Annu. Rev. Neurosci. 27, 509-547. - PubMed
-
- Sutton, R. B., Davletov, B. A., Berghuis, A. M., Südhof, T. C. & Sprang, S. R. (1995) Cell 80, 929-938. - PubMed
-
- Fernandez, I., Arac, D., Ubach, J., Gerber, S. H., Shin, O. H., Gao, Y., Anderson, R. G., Südhof, T. C. & Rizo, J. (2001) Neuron 32, 1057-1069. - PubMed
-
- Shao, X., Davletov, B. A., Sutton, R. B., Südhof, T. C. & Rizo, J. (1996) Science 273, 248-251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

