Leucine-rich repeat kinase 2 (LRRK2) interacts with parkin, and mutant LRRK2 induces neuronal degeneration
- PMID: 16352719
- PMCID: PMC1317945
- DOI: 10.1073/pnas.0508052102
Leucine-rich repeat kinase 2 (LRRK2) interacts with parkin, and mutant LRRK2 induces neuronal degeneration
Abstract
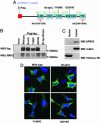
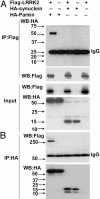
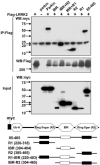
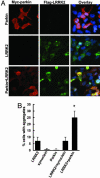
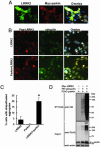
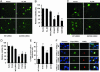
Parkinson's disease (PD) is a disorder of movement, cognition, and emotion, and it is characterized pathologically by neuronal degeneration with Lewy bodies, which are cytoplasmic inclusion bodies containing deposits of aggregated proteins. Most PD cases appear to be sporadic, but genetic forms of the disease, caused by mutations in alpha-synuclein, parkin, and other genes, have helped elucidate pathogenesis. Mutations in leucine-rich repeat kinase 2 (LRRK2) cause autosomal-dominant Parkinsonism with clinical features of PD and with pleomorphic pathology including deposits of aggregated protein. To study expression and interactions of LRRK2, we synthesized cDNAs and generated expression constructs coding for human WT and mutant LRRK2 proteins. Expression of full-length LRRK2 in cells in culture suggests that the protein is predominately cytoplasmic, as is endogenous protein by subcellular fractionation. Using coimmunoprecipitation, we find that LRRK2, expressed in cells in culture, interacts with parkin but not with alpha-synuclein, DJ-1, or tau. A small proportion of the cells overexpressing LRRK2 contain protein aggregates, and this proportion is greatly increased by coexpression of parkin. In addition, parkin increases ubiquitination of aggregated protein. Also, mutant LRRK2 causes neuronal degeneration in both SH-SY5Y cells and primary neurons. This cell model may be useful for studies of PD cellular pathogenesis and therapeutics. These findings suggest a gain-of-function mechanism in the pathogenesis of LRRK2-linked PD and suggest that LRRK2 may be involved in a pathogenic pathway with other PD-related proteins such as parkin, which may help illuminate both familial and sporadic PD.
Figures
Similar articles
-
LRRK2 and parkin immunoreactivity in multiple system atrophy inclusions.Acta Neuropathol. 2008 Dec;116(6):639-46. doi: 10.1007/s00401-008-0446-3. Epub 2008 Oct 21. Acta Neuropathol. 2008. PMID: 18936941
-
Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson's-disease-related mutant alpha-synuclein.Neuron. 2009 Dec 24;64(6):807-27. doi: 10.1016/j.neuron.2009.11.006. Neuron. 2009. PMID: 20064389 Free PMC article.
-
LRRK2 and neurodegeneration.Acta Neuropathol. 2009 Mar;117(3):227-46. doi: 10.1007/s00401-008-0478-8. Epub 2009 Jan 14. Acta Neuropathol. 2009. PMID: 19142648 Review.
-
Role of autophagy in G2019S-LRRK2-associated neurite shortening in differentiated SH-SY5Y cells.J Neurochem. 2008 May;105(3):1048-56. doi: 10.1111/j.1471-4159.2008.05217.x. Epub 2008 Jan 7. J Neurochem. 2008. PMID: 18182054 Free PMC article.
-
[Clinical molecular genetics for PARK8 (LRRK2)].Brain Nerve. 2007 Aug;59(8):839-50. Brain Nerve. 2007. PMID: 17713120 Review. Japanese.
Cited by
-
Coordinate Regulation of Neurite Outgrowth by LRRK2 and Its Interactor, Rab5.Exp Neurobiol. 2010 Sep;19(2):97-105. doi: 10.5607/en.2010.19.2.97. Epub 2010 Sep 30. Exp Neurobiol. 2010. PMID: 22110348 Free PMC article.
-
Genetic analysis of Parkinson's disease-linked leucine-rich repeat kinase 2.Biochem Soc Trans. 2012 Oct;40(5):1042-6. doi: 10.1042/BST20120112. Biochem Soc Trans. 2012. PMID: 22988862 Free PMC article. Review.
-
Roles of the Drosophila LRRK2 homolog in Rab7-dependent lysosomal positioning.Hum Mol Genet. 2012 Mar 15;21(6):1350-63. doi: 10.1093/hmg/ddr573. Epub 2011 Dec 13. Hum Mol Genet. 2012. PMID: 22171073 Free PMC article.
-
LRRK2 G2019S mutation induces dendrite degeneration through mislocalization and phosphorylation of tau by recruiting autoactivated GSK3ß.J Neurosci. 2010 Sep 29;30(39):13138-49. doi: 10.1523/JNEUROSCI.1737-10.2010. J Neurosci. 2010. PMID: 20881132 Free PMC article.
-
Sequence conservation between porcine and human LRRK2.Mol Biol Rep. 2009 Feb;36(2):237-43. doi: 10.1007/s11033-007-9172-5. Epub 2007 Nov 3. Mol Biol Rep. 2009. PMID: 17978862
References
-
- Dauer, W. & Przedborski, S. (2003) Neuron 39, 889-909. - PubMed
-
- Forno, L. S. (1996) J. Neuropathol. Exp. Neurol. 55, 259-272. - PubMed
-
- Mouradian, M. M. (2002) Neurology 58, 179-185. - PubMed
-
- Dawson, T. M. & Dawson, V. L. (2003) Science 302, 819-822. - PubMed
-
- Taylor, J. P., Hardy, J. & Fischbeck, K. H. (2002) Science 296, 1991-1995. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

