Galactocerebrosidase-deficient oligodendrocytes maintain stable central myelin by exogenous replacement of the missing enzyme in mice
- PMID: 16352725
- PMCID: PMC1317926
- DOI: 10.1073/pnas.0506473102
Galactocerebrosidase-deficient oligodendrocytes maintain stable central myelin by exogenous replacement of the missing enzyme in mice
Abstract
Globoid cell leukodystrophy (GLD) is a lysosomal storage disease caused by genetic deficiency of galactocerebrosidase (GALC) activity. Failure in catalyzing the degradation of its major substrate, galactocerebroside, in oligodendrocytes (OLs) and Schwann cells leads to death of these myelinating cells, progressive demyelination, and early demise of GLD patients. Transplantation of bone marrow cells and umbilical cord blood have been attempted as a means of enzyme replacement and have shown limited success. It remains unknown whether or how these therapies support survival of GALC-deficient OLs and myelin maintenance. We report that, upon transplantation, GALC-deficient OLs from the twitcher mouse, a model of GLD, achieved widespread myelination in the brain and spinal cord of the myelin-deficient shiverer mouse, which was preserved for the life of the host. GALC immunohistochemistry showed direct evidence for GALC transfer from the shiverer environment to the engrafted mutant OLs in vivo. These findings suggest that the mutant OLs can internalize exogenous GALC and maintain stable myelin, demonstrating that exogenous enzyme replacement will be a key strategy in the therapy of GLD.
Figures
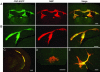

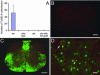


Similar articles
-
CNS-targeted AAV5 gene transfer results in global dispersal of vector and prevention of morphological and function deterioration in CNS of globoid cell leukodystrophy mouse model.Mol Genet Metab. 2011 Aug;103(4):367-77. doi: 10.1016/j.ymgme.2011.05.005. Epub 2011 May 12. Mol Genet Metab. 2011. PMID: 21620749
-
Enzyme replacement therapy of a novel humanized mouse model of globoid cell leukodystrophy.Exp Neurol. 2015 Sep;271:36-45. doi: 10.1016/j.expneurol.2015.04.020. Epub 2015 May 6. Exp Neurol. 2015. PMID: 25956830
-
GALC transduction leads to morphological improvement of the twitcher oligodendrocytes in vivo.Mol Genet Metab. 2005 Apr;84(4):332-43. doi: 10.1016/j.ymgme.2004.12.007. Epub 2005 Jan 24. Mol Genet Metab. 2005. PMID: 15781194
-
Myelin repair by transplantation of myelin-forming cells in globoid cell leukodystrophy.J Neurosci Res. 2016 Nov;94(11):1195-202. doi: 10.1002/jnr.23909. Epub 2016 Aug 25. J Neurosci Res. 2016. PMID: 27557886 Review.
-
Mechanisms of demyelination and neurodegeneration in globoid cell leukodystrophy.Glia. 2021 Oct;69(10):2309-2331. doi: 10.1002/glia.24008. Epub 2021 Apr 14. Glia. 2021. PMID: 33851745 Free PMC article. Review.
Cited by
-
Human iPSC-derived astrocytes generated from donors with globoid cell leukodystrophy display phenotypes associated with disease.PLoS One. 2022 Aug 3;17(8):e0271360. doi: 10.1371/journal.pone.0271360. eCollection 2022. PLoS One. 2022. PMID: 35921286 Free PMC article.
-
Preclinical studies in Krabbe disease: A model for the investigation of novel combination therapies for lysosomal storage diseases.Mol Ther. 2023 Jan 4;31(1):7-23. doi: 10.1016/j.ymthe.2022.09.017. Epub 2022 Oct 4. Mol Ther. 2023. PMID: 36196048 Free PMC article. Review.
-
Adult Krabbe Disease That Was Successfully Treated with Intravenous Immunoglobulin.Intern Med. 2021 Apr 15;60(8):1283-1286. doi: 10.2169/internalmedicine.6094-20. Epub 2020 Nov 16. Intern Med. 2021. PMID: 33191329 Free PMC article.
-
The role of AMPK in psychosine mediated effects on oligodendrocytes and astrocytes: implication for Krabbe disease.J Neurochem. 2008 Jun;105(5):1820-33. doi: 10.1111/j.1471-4159.2008.05279.x. Epub 2008 Feb 4. J Neurochem. 2008. PMID: 18248608 Free PMC article.
-
A microglial hypothesis of globoid cell leukodystrophy pathology.J Neurosci Res. 2016 Nov;94(11):1049-61. doi: 10.1002/jnr.23773. J Neurosci Res. 2016. PMID: 27638591 Free PMC article. Review.
References
-
- Wenger, D. A., Suzuki, K., Suzuki, Y. & Suzuki, K. (2001) in The Metabolic and Molecular Bases of Inherited Disease, eds. Scriver, C. R., Beaudet, A. L., Sly, W. S., Valle, D., Childs, B., Kinzler, K. W. & Vogelstein, B. (McGraw-Hill, New York), pp. 3669-3694.
-
- Duchen, L. W., Eicher, E. M., Jacobs, J. M., Scaravilli, F. & Teixeira, F. (1980) Brain 103, 695-710. - PubMed
-
- Kobayashi, T., Yamanaka, T., Jacobs, J. M., Teixeira, F. & Suzuki, K. (1980) Brain Res. 202, 479-483. - PubMed
-
- Sakai, N., Inui, K., Tatsumi, N., Fukushima, H., Nishigaki, T., Taniike, M., Nishimoto, J., Tsukamoto, H., Yanagihara, I., Ozono, K., et al. (1996) J. Neurochem. 66, 1118-1124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

