Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation
- PMID: 16352730
- PMCID: PMC1317903
- DOI: 10.1073/pnas.0504343102
Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation
Abstract
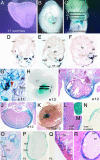
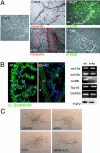
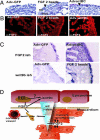
Vitamin A signals play critical roles during embryonic development. In particular, heart morphogenesis depends on vitamin A signals mediated by the retinoid X receptor alpha (RXRalpha), as the systemic mutation of this receptor results in thinning of the myocardium and embryonic lethality. However, the molecular and cellular mechanisms controlled by RXRalpha signaling in this process are unclear, because a myocardium-restricted RXRalpha mutation does not perturb heart morphogenesis. Here, we analyze a series of tissue-restricted mutations of the RXRalpha gene in the cardiac neural crest, endothelial, and epicardial lineages, and we show that RXRalpha signaling in the epicardium is required for proper cardiac morphogenesis. Moreover, we detect an additional phenotype of defective coronary arteriogenesis associated with RXRalpha deficiency and identify a retinoid-dependent Wnt signaling pathway that cooperates in epicardial epithelial-to-mesenchymal transformation.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

