RscA, a member of the MDR1 family of transporters, is repressed by CovR and required for growth of Streptococcus pyogenes under heat stress
- PMID: 16352823
- PMCID: PMC1317578
- DOI: 10.1128/JB.188.1.77-85.2006
RscA, a member of the MDR1 family of transporters, is repressed by CovR and required for growth of Streptococcus pyogenes under heat stress
Abstract
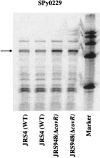
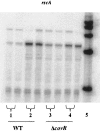
The ability of Streptococcus pyogenes (group A streptococcus [GAS]) to respond to changes in environmental conditions is essential for this gram-positive organism to successfully cause disease in its human host. The two-component system CovRS controls expression of about 15% of the GAS genome either directly or indirectly. In most operons studied, CovR acts as a repressor. We previously linked CovRS to the GAS stress response by showing that the sensor kinase CovS is required to inactivate the response regulator CovR so that GAS can grow under conditions of heat, acid, and salt stress. Here, we sought to identify CovR-repressed genes that are required for growth under stress. To do this, global transcription profiles were analyzed by microarrays following exposure to increased temperature (40 degrees C) and decreased pH (pH 6.0). The CovR regulon in an M type 6 strain of GAS was also examined by global transcriptional analysis. We identified a gene, rscA (regulated by stress and Cov), whose transcription was confirmed to be repressed by CovR and activated by heat and acid. RscA is a member of the MDR1 family of ABC transporters, and we found that it is required for growth of GAS at 40 degrees C but not at pH 6.0. Thus, for GAS to grow at 40 degrees C, CovR repression must be alleviated so that rscA can be transcribed to allow the production of this potential exporter. Possible explanations for the thermoprotective role of RscA in this pathogen are discussed.
Figures





References
-
- Abrams, A., and C. Jensen. 1984. Altered expression of the H+ ATPase in Streptococcus faecalis membranes. Biochem. Biophys. Res. Commun. 122:151-157. - PubMed
-
- Adamcik, J., V. Viglasky, F. Valle, M. Antalik, D. Podhradsky, and G. Dietler. 2002. Effect of bacteria growth temperature on the distribution of supercoiled DNA and its thermal stability. Electrophoresis 23:3300-3309. - PubMed
-
- Bang, I. S., J. P. Audia, Y. K. Park, and J. W. Foster. 2002. Autoinduction of the OmpR response regulator by acid shock and control of the Salmonella enterica acid tolerance response. Mol. Microbiol. 44:1235-1250. - PubMed
-
- Beres, S. B., G. L. Sylva, K. D. Barbian, B. Lei, J. S. Hoff, N. D. Mammarella, M. Y. Liu, J. C. Smoot, S. F. Porcella, L. D. Parkins, D. S. Campbell, T. M. Smith, J. K. McCormick, D. Y. Leung, P. M. Schlievert, and J. M. Musser. 2002. Genome sequence of a serotype M3 strain of group A streptococcus: phage-encoded toxins, the high-virulence phenotype, and clone emergence. Proc. Natl. Acad. Sci. USA 99:10078-10083. - PMC - PubMed
-
- Bhriain, N. N., C. J. Dorman, and C. F. Higgins. 1989. An overlap between osmotic and anaerobic stress responses: a potential role for DNA supercoiling in the coordinate regulation of gene expression. Mol. Microbiol. 3:933-942. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

