The Pseudomonas aeruginosa lipid A deacylase: selection for expression and loss within the cystic fibrosis airway
- PMID: 16352835
- PMCID: PMC1317579
- DOI: 10.1128/JB.188.1.191-201.2006
The Pseudomonas aeruginosa lipid A deacylase: selection for expression and loss within the cystic fibrosis airway
Abstract
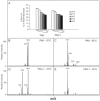
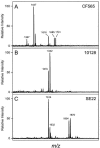
Lipopolysaccharide (LPS) is the major surface component of gram-negative bacteria, and a component of LPS, lipid A, is recognized by the innate immune system through the Toll-like receptor 4/MD-2 complex. Pseudomonas aeruginosa, an environmental gram-negative bacterium that opportunistically infects the respiratory tracts of patients with cystic fibrosis (CF), can synthesize various structures of lipid A. Lipid A from P. aeruginosa strains isolated from infants with CF has a specific structure that includes the removal of the 3 position 3-OH C10 fatty acid. Here we demonstrate increased expression of the P. aeruginosa lipid A 3-O-deacylase (PagL) in isolates from CF infants compared to that in environmental isolates. PagL activity was increased in environmental isolates by growth in medium limited for magnesium and decreased by growth at low temperature in laboratory-adapted strains of P. aeruginosa. P. aeruginosa PagL was shown to be an outer membrane protein by isopycnic density gradient centrifugation. Heterologous expression of P. aeruginosa pagL in Salmonella enterica serovar Typhimurium and Escherichia coli resulted in removal of the 3-OH C14 fatty acid from lipid A, indicating that P. aeruginosa PagL recognizes either 3-OH C10 or 3-OH C14. Finally, deacylated lipid A species were not observed in some clinical P. aeruginosa isolates from patients with severe pulmonary disease, suggesting that loss of PagL function can occur during long-term adaptation to the CF airway.
Figures


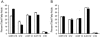

Similar articles
-
Dissemination of lipid A deacylases (pagL) among gram-negative bacteria: identification of active-site histidine and serine residues.J Biol Chem. 2005 Mar 4;280(9):8248-59. doi: 10.1074/jbc.M414235200. Epub 2004 Dec 20. J Biol Chem. 2005. PMID: 15611102
-
Inhibition of Salmonella enterica serovar Typhimurium lipopolysaccharide deacylation by aminoarabinose membrane modification.J Bacteriol. 2005 Apr;187(7):2448-57. doi: 10.1128/JB.187.7.2448-2457.2005. J Bacteriol. 2005. PMID: 15774888 Free PMC article.
-
A PhoP/PhoQ-induced Lipase (PagL) that catalyzes 3-O-deacylation of lipid A precursors in membranes of Salmonella typhimurium.J Biol Chem. 2001 Mar 23;276(12):9083-92. doi: 10.1074/jbc.M010730200. Epub 2000 Dec 6. J Biol Chem. 2001. PMID: 11108722
-
Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: an evolutionary perspective.Nat Rev Microbiol. 2012 Dec;10(12):841-51. doi: 10.1038/nrmicro2907. Epub 2012 Nov 13. Nat Rev Microbiol. 2012. PMID: 23147702 Review.
-
Microevolution of Pseudomonas aeruginosa to a chronic pathogen of the cystic fibrosis lung.Curr Top Microbiol Immunol. 2013;358:91-118. doi: 10.1007/82_2011_199. Curr Top Microbiol Immunol. 2013. PMID: 22311171 Review.
Cited by
-
Current concepts: host-pathogen interactions in cystic fibrosis airways disease.Eur Respir Rev. 2014 Sep;23(133):320-32. doi: 10.1183/09059180.00006113. Eur Respir Rev. 2014. PMID: 25176968 Free PMC article. Review.
-
Presence of 2-hydroxymyristate on endotoxins is associated with death in neonates with Enterobacter cloacae complex septic shock.iScience. 2021 Jul 30;24(8):102916. doi: 10.1016/j.isci.2021.102916. eCollection 2021 Aug 20. iScience. 2021. PMID: 34409274 Free PMC article.
-
Structural biology of membrane-intrinsic beta-barrel enzymes: sentinels of the bacterial outer membrane.Biochim Biophys Acta. 2008 Sep;1778(9):1881-96. doi: 10.1016/j.bbamem.2007.07.021. Epub 2007 Aug 11. Biochim Biophys Acta. 2008. PMID: 17880914 Free PMC article. Review.
-
The Pseudomonas aeruginosa PhoP-PhoQ two-component regulatory system is induced upon interaction with epithelial cells and controls cytotoxicity and inflammation.Infect Immun. 2012 Sep;80(9):3122-31. doi: 10.1128/IAI.00382-12. Epub 2012 Jun 18. Infect Immun. 2012. PMID: 22710876 Free PMC article.
-
Cross-family transfer of the Arabidopsis cell-surface immune receptor LORE to tomato confers sensing of 3-hydroxylated fatty acids and enhanced disease resistance.Mol Plant Pathol. 2024 Sep;25(9):e70005. doi: 10.1111/mpp.70005. Mol Plant Pathol. 2024. PMID: 39235143 Free PMC article.
References
-
- Berger, M. 2002. Inflammatory mediators in cystic fibrosis lung disease. Allergy Asthma Proc. 23:19-25. - PubMed
-
- Bishop, R. E. 2005. Fundamentals of endotoxin structure and function. Contrib. Microbiol. 12:1-27. - PubMed
-
- Bishop, R. E., S. H. Kim, and A. El Zoeiby. 2005. Role of lipid A palmitoylation in bacterial pathogenesis. J. Endotoxin Res. 11:174-180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

