Solution structure of a novel tryptophan-rich peptide with bidirectional antimicrobial activity
- PMID: 16352849
- PMCID: PMC1317575
- DOI: 10.1128/JB.188.1.328-334.2006
Solution structure of a novel tryptophan-rich peptide with bidirectional antimicrobial activity
Abstract
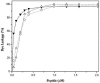
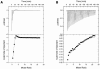
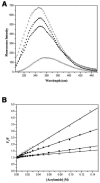
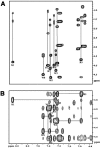
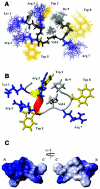
Trp-rich antimicrobial peptides play important roles in the host innate defense mechanisms of many plants, insects, and mammals. A new type of Trp-rich peptide, Ac-KWRRWVRWI-NH(2), designated Pac-525, was found to possess improved activity against both gram-positive and -negative bacteria. We have determined that the solution structures of Pac-525 bound to membrane-mimetic sodium dodecyl sulfate (SDS) micelles. The SDS micelle-bound structure of Pac-525 adopts an alpha-helical segment at residues Trp2, Arg3, and Arg4. The positively charged residues are clustered together to form a hydrophilic patch. The three hydrophobic residues Trp2, Val6, and Ile9 form a hydrophobic core. The surface electrostatic potential map indicates the three tryptophan indole rings are packed against the peptide backbone and form an amphipathic structure. Moreover, the reverse sequence of Pac-525, Ac-IWRVWRRWK-NH(2), designated Pac-525(rev), also demonstrates similar antimicrobial activity and structure in membrane-mimetic micelles and vesicles. A variety of biophysical and biochemical methods, including circular dichroism, fluorescence spectroscopy, and microcalorimetry, were used to show that Pac-525 interacted strongly with negatively charged phospholipid vesicles and induced efficient dye release from these vesicles, suggesting that the antimicrobial activity of Pac-525 may be due to interactions with bacterial membranes.
Figures
References
-
- Allen, T. M. (ed.). 1984. Calcein as a tool in liposome methodology. CRC Press, Boca Raton, Fla.
-
- Andreu, D., and L. Rivas. 1998. Animal antimicrobial peptides: an overview. Biopolymers 47:415-433. - PubMed
-
- Blondelle, S., and K. Lohner. 2000. Combinatorial libraries: a tool to design antimicrobial and antifungal peptide analogues having lytic specificities for structure-activity relationship studies. Biopolymers 55:74-87. - PubMed
-
- Brock, T. D. 1974. Biology of microorganisms, 2nd ed. Prentice-Hall Inc., Englewood Cliffs, N.J.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

