Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage
- PMID: 16354677
- PMCID: PMC1317617
- DOI: 10.1128/MCB.26.1.28-38.2006
Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage
Abstract
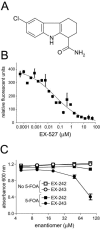
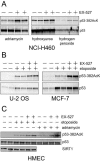
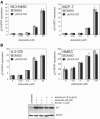
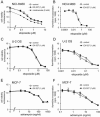
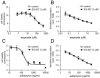
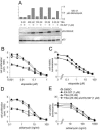
Human SIRT1 is an enzyme that deacetylates the p53 tumor suppressor protein and has been suggested to modulate p53-dependent functions including DNA damage-induced cell death. In this report, we used EX-527, a novel, potent, and specific small-molecule inhibitor of SIRT1 catalytic activity to examine the role of SIRT1 in p53 acetylation and cell survival after DNA damage. Treatment with EX-527 dramatically increased acetylation at lysine 382 of p53 after different types of DNA damage in primary human mammary epithelial cells and several cell lines. Significantly, inhibition of SIRT1 catalytic activity by EX-527 had no effect on cell growth, viability, or p53-controlled gene expression in cells treated with etoposide. Acetyl-p53 was also increased by the histone deacetylase (HDAC) class I/II inhibitor trichostatin A (TSA). EX-527 and TSA acted synergistically to increase acetyl-p53 levels, confirming that p53 acetylation is regulated by both SIRT1 and HDACs. While TSA alone reduced cell survival after DNA damage, the combination of EX-527 and TSA had no further effect on cell viability and growth. These results show that, although SIRT1 deacetylates p53, this does not play a role in cell survival following DNA damage in certain cell lines and primary human mammary epithelial cells.
Figures

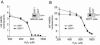
References
-
- Appella, E., and C. W. Anderson. 2001. Post-translational modifications and activation of p53 by genotoxic stresses. Eur. J. Biochem. 268:2764-2772. - PubMed
-
- Barlev, N. A., L. Liu, N. H. Chehab, K. Mansfield, K. G. Harris, T. D. Halazonetis, and S. L. Berger. 2001. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol. Cell 8:1243-1254. - PubMed
-
- Bitterman, K. J., R. M. Anderson, H. Y. Cohen, M. Latorre-Esteves, and D. A. Sinclair. 2002. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J. Biol. Chem. 277:45099-45107. - PubMed
-
- Blander, G., and L. Guarente. 2004. The Sir2 family of protein deacetylases. Annu. Rev. Biochem. 73:417-435. - PubMed
-
- Bordone, L., and L. Guarente. 2005. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat. Rev. Mol. Cell Biol. 6:298-305. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
