Mitogen-activated protein kinase cascade-mediated histone H3 phosphorylation is critical for telomerase reverse transcriptase expression/telomerase activation induced by proliferation
- PMID: 16354694
- PMCID: PMC1317632
- DOI: 10.1128/MCB.26.1.230-237.2006
Mitogen-activated protein kinase cascade-mediated histone H3 phosphorylation is critical for telomerase reverse transcriptase expression/telomerase activation induced by proliferation
Abstract
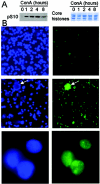
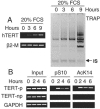
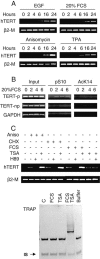
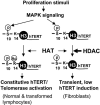
Telomerase activity and telomerase reverse transcriptase (hTERT), the key component of the telomerase complex, are tightly proliferation regulated in normal and malignant cells both in vitro and in vivo; however, underlying mechanisms are unclear. In the present study, we identified mitogen-activated protein kinase (MAPK) cascade-mediated histone H3 ser10 phosphorylation to be a molecular link between proliferation and induction of hTERT/telomerase activity. In normal human T lymphocytes and fibroblasts, growth or stress stimuli known to drive H3 phosphorylation through the MAPK signaling induce hTERT expression and/or telomerase activity that was preceded by phosphorylated histone H3 (ser10) at the hTERT promoter. Blockade of the MAPK-triggered H3 phosphorylation significantly abrogates hTERT induction and ser10 phosphorylation at this promoter. However, H3 ser10 phosphorylation alone resulted in low, transient hTERT induction, as seen in fibroblasts, whereas H3 phosphorylation followed by its acetylation at lys14 robustly trans-activated the hTERT gene accompanying constitutive telomerase activity in normal and malignant T cells. H3 acetylation without phosphorylation similarly exerted weak effects on hTERT expression. These results define H3 phosphorylation as a key to hTERT transactivation induced by proliferation and reveal a fundamental mechanism for telomerase regulation in both normal human cells and transformed T cells.
Figures

References
-
- Blasco, M. A. 2003. Telomeres and cancer: a tale with many endings. Curr. Opin. Genet. Dev. 13:70-76. - PubMed
-
- Chadee, D. N., M. J. Hendzel, C. P. Tylipski, C. D. Allis, D. P. Bazett-Jones, J. A. Wright, and J. R. Davie. 1999. Increased Ser-10 phosphorylation of histone H3 in mitogen-stimulated and oncogene-transformed mouse fibroblasts. J. Biol. Chem. 274:24914-24920. - PubMed
-
- Chadeneau, C., P. Siegel, C. B. Harley, W. J. Muller, and S. Bacchetti. 1995. Telomerase activity in normal and malignant murine tissues. Oncogene 11:893-898. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
