Extrasynaptic GABAA receptors of thalamocortical neurons: a molecular target for hypnotics
- PMID: 16354909
- PMCID: PMC6726038
- DOI: 10.1523/JNEUROSCI.2679-05.2005
Extrasynaptic GABAA receptors of thalamocortical neurons: a molecular target for hypnotics
Abstract
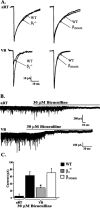
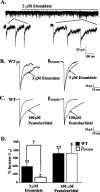
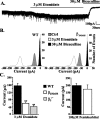
Among hypnotic agents that enhance GABAA receptor function, etomidate is unusual because it is selective for beta2/beta3 compared with beta1 subunit-containing GABAA receptors. Mice incorporating an etomidate-insensitive beta2 subunit (beta(2N265S)) revealed that beta2 subunit-containing receptors mediate the enhancement of slow-wave activity (SWA) by etomidate, are required for the sedative, and contribute to the hypnotic actions of this anesthetic. Although the anatomical location of the beta2-containing receptors that mediate these actions is unknown, the thalamus is implicated. We have characterized GABAA receptor-mediated neurotransmission in thalamic nucleus reticularis (nRT) and ventrobasalis complex (VB) neurons of wild-type, beta2(-/-), and beta(2N265S) mice. VB but not nRT neurons exhibit a large GABA-mediated tonic conductance that contributes approximately 80% of the total GABAA receptor-mediated transmission. Consequently, although etomidate enhances inhibition in both neuronal types, the effect of this anesthetic on the tonic conductance of VB neurons is dominant. The GABA-enhancing actions of etomidate in VB but not nRT neurons are greatly suppressed by the beta(2N265S) mutation. The hypnotic THIP (Gaboxadol) induces SWA and at low, clinically relevant concentrations (30 nM to 3 microM) increases the tonic conductance of VB neurons, with no effect on VB or nRT miniature IPSCs (mIPSCs) or on the holding current of nRT neurons. Zolpidem, which has no effect on SWA, prolongs VB mIPSCs but is ineffective on the phasic and tonic conductance of nRT and VB neurons, respectively. Collectively, these findings suggest that enhancement of extrasynaptic inhibition in the thalamus may contribute to the distinct sleep EEG profiles of etomidate and THIP compared with zolpidem.
Figures

References
-
- Alkire MT, Haier RJ, Fallon JH (2000) Toward a unified theory of narcosis: brain imaging evidence for a thalamocortical switch as the neurophysiologic basis of anesthetic-induced unconsciousness. Conscious Cogn 9: 370–386. - PubMed
-
- Belelli D, Lambert JJ (2005) Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci 6: 565–575. - PubMed
-
- Belelli D, Pistis M, Peters JA, Lambert JJ (1999) General anaesthetic action at transmitter-gated inhibitory amino acid receptors. Trends Pharmacol Sci 20: 496–502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
