Neurosteroid access to the GABAA receptor
- PMID: 16354918
- PMCID: PMC6726021
- DOI: 10.1523/JNEUROSCI.4173-05.2005
Neurosteroid access to the GABAA receptor
Abstract
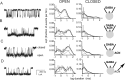
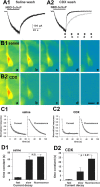
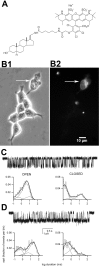
GABAA receptors are a pivotal inhibitory influence in the nervous system, and modulators of the GABAA receptor are important anesthetics, sedatives, anticonvulsants, and anxiolytics. Current views of receptor modulation suggest that many exogenous drugs access and bind to an extracellular receptor domain. Using novel synthetic steroid analogs, we examined the access route for neuroactive steroids, potent GABAA receptor modulators also produced endogenously. Tight-seal recordings, in which direct aqueous drug access to receptor was prevented, demonstrated that steroids can reach the receptor either through plasma membrane lateral diffusion or through intracellular routes. A fluorescent neuroactive steroid accumulated intracellularly, but recordings from excised patches indicated that the intracellular reservoir is not necessary for receptor modulation, although it can apparently equilibrate with the plasma membrane within seconds. A membrane impermeant neuroactive steroid modulated receptor activity only when applied to the inner membrane leaflet, demonstrating that the steroid does not access an extracellular modulatory site. Thus, neuroactive steroids do not require direct aqueous access to the receptor, and membrane accumulation is required for receptor modulation.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
