Smad3-deficient chondrocytes have enhanced BMP signaling and accelerated differentiation
- PMID: 16355269
- PMCID: PMC2649698
- DOI: 10.1359/JBMR.050911
Smad3-deficient chondrocytes have enhanced BMP signaling and accelerated differentiation
Abstract
Smad3 deficiency accelerates chondrocyte maturation and leads to osteoarthritis. Primary chondrocytes without Smad3 lack compensatory increases of TGF-beta signaling factors, but BMP-related gene expression is increased. Smad2 or Smad3 overexpression and BMP blockade abrogate accelerated maturation in Smad3-/- chondrocytes. BMP signaling is increased in TGF-beta deficiency and is required for accelerated chondrocyte maturation.
Introduction: Disruption of TGF-beta signaling results in accelerated chondrocyte maturation and leads to postnatal dwarfism and premature osteoarthritis. The mechanisms involved in this process were studied using in vitro murine chondrocyte cultures.
Materials and methods: Primary chondrocytes were isolated from the sterna of neonatal wildtype and Smad3-/- mice. Expressions of maturational markers, as well as genes involved in TGF-beta and BMP signaling were examined. Chondrocytes were treated with TGF-beta and BMP-2, and effects on maturation-related genes and BMP/TGF-beta responsive reporters were examined. Recombinant noggin or retroviral vectors expressing Smad2 or Smad3 were added to the cultures.
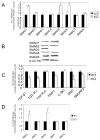
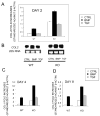
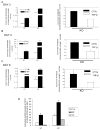
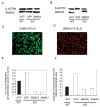
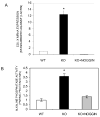
Results: Expression of colX and other maturational markers was markedly increased in Smad3-/- chondrocytes. Smad3-/- chondrocytes lacked compensatory increases in Smad2, Smad4, TGFRII, Sno, or Smurf2 and had reduced expression of TGF-beta1 and TGFRI. In contrast, Smad1, Smad5, BMP2, and BMP6 expression was increased, suggesting a shift from TGF-beta toward BMP signaling. In Smad3-/- chondrocytes, alternative TGF-beta signaling pathways remained responsive, as shown by luciferase assays. These non-Smad3-dependent TGF-beta pathways reduced colX expression and alkaline phosphatase activity in TGF-beta-treated Smad3-/- cultures, but only partially. In contrast, Smad3-/- chondrocytes were more responsive to BMP-2 treatment and had increased colX expression, phosphoSmads 1, 5, and 8 levels, and luciferase reporter activity. Overexpression of both Smad2 and Smad3 blocked spontaneous maturation in Smad3-deficient chondrocytes. Maturation was also abrogated by the addition of noggin, an extracellular BMP inhibitor.
Conclusions: These findings show a key role for BMP signaling during the chondrocyte maturation, occurring with loss of TGF-beta signaling with important implications for osteoarthritis and cartilage diseases.
Conflict of interest statement
The authors have no conflict of interest.
Figures


References
-
- Massague J, Chen Y. Controlling TGF-beta signaling. Genes Dev. 2000;14:627–640. - PubMed
-
- D’Angelo M, Yan Z, Nooreyazdan M, Pacifici M, Sarment DS, Billings PC, Leboy PS. MMP-13 is induced during chondrocyte hypertrophy. J Cell Biochem. 2000;77:678–693. - PubMed
-
- O’Keefe R, Puzas JE, Loveys L, Hicks DG, Rosier RN. Analysis of type II and type X collagen synthesis in cultured growth plate chondrocytes by in situ hybridization: Rapid induction of type X collagen in culture. J Bone Miner Res. 1994;9:1713–1722. - PubMed
-
- Volk SW, Lu Valle P, Leask T, Leboy PS. A BMP responsive transcriptional region in the chicken type X collagen gene. J Bone Miner Res. 1998;13:1521–1529. - PubMed
-
- Gerstenfeld LC, Shapiro FD. Expression of bone-specific genes by hypertrophic chondrocytes: Implication of the complex functions of the hypertrophic chondrocyte during endochondral bone development. J Cell Biochem. 1996;62:1–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

