Chemical modification and irreversible inhibition of striatal A2a adenosine receptors
- PMID: 1635550
- PMCID: PMC3429947
Chemical modification and irreversible inhibition of striatal A2a adenosine receptors
Abstract
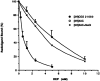
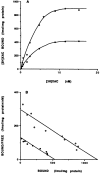
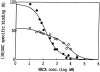
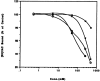
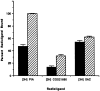
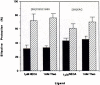
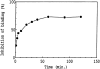
The ligand recognition site of A2a-adenosine receptors in rabbit striatal membranes was probed using non-site-directed labeling reagents and specific affinity labels. Exposure of membranes to diethylpyrocarbonate at a concentration of 2.5 mM, followed by washing, was found to inhibit the binding of [3H]CGS 21680 and [3H]xanthine amine congener to A2a receptors, by 86 and 30%, respectively. Protection from diethylpyrocarbonate inactivation by an adenosine receptor agonist, 5'-N-ethylcarboxamidoadenosine, and an antagonist, theophylline, suggested the presence of two histidyl residues on the receptor, one associated with agonist binding and the other with antagonist binding. Binding of [3H]CGS 21680 or [3H]xanthine amine congener was partially restored after incubation with 250 mM hydroxylamine, further supporting histidine as the modification site. Preincubation with disulfide-reactive reagents, dithiothreitol or sodium dithionite, at greater than 5 mM inhibited radioligand binding, indicating the presence of essential disulfide bridges in A2a receptors, whereas the concentration of mercaptoethanol required to inhibit binding was greater than 50 mM. A number of isothiocyanate-bearing affinity labels derived from the A2a-selective agonist 2-[(2-aminoethylamino) carbonylethylphenylethylamino]-5'-N- ethylcarboxamidoadenosine (APEC) were synthesized and found to inhibit A2a receptor binding in rabbit and bovine striatal membranes. Binding to rabbit A1 receptors was not inhibited. Preincubation with the affinity label 4-isothiocyanatophenylaminothiocarbonyl-APEC (100 nM) diminished the Bmax for [3H]CGS 21680 binding by 71%, and the Kd was unaffected, suggesting a direct modification of the ligand binding site. Reversal of 4-isothiocyanatophenylaminothiocarbonyl-APEC inhibition of [3H]CGS 21680 binding with hydroxylamine suggested that the site of modification by the isothiocyanate is a cysteine residue. A bromoacetyl derivative of APEC was ineffective as an affinity label at submicromolar concentrations.
Figures



Similar articles
-
Evidence for high-affinity binding sites for the adenosine A2A receptor agonist [3H] CGS 21680 in the rat hippocampus and cerebral cortex that are different from striatal A2A receptors.Naunyn Schmiedebergs Arch Pharmacol. 1996 Feb;353(3):261-71. doi: 10.1007/BF00168627. Naunyn Schmiedebergs Arch Pharmacol. 1996. PMID: 8692280
-
[3H]CGS 21680, a selective A2 adenosine receptor agonist directly labels A2 receptors in rat brain.J Pharmacol Exp Ther. 1989 Dec;251(3):888-93. J Pharmacol Exp Ther. 1989. PMID: 2600819
-
Role of cysteine residues of rat A2a adenosine receptors in agonist binding.Biochim Biophys Acta. 1997 Feb 21;1324(1):159-70. doi: 10.1016/s0005-2736(96)00223-4. Biochim Biophys Acta. 1997. PMID: 9059509
-
Binding of the radioligand [3H]-SCH 58261, a new non-xanthine A2A adenosine receptor antagonist, to rat striatal membranes.Br J Pharmacol. 1996 Apr;117(7):1381-6. doi: 10.1111/j.1476-5381.1996.tb15296.x. Br J Pharmacol. 1996. PMID: 8730729 Free PMC article.
-
Characterization of human striatal A2-adenosine receptors using radioligand binding and photoaffinity labeling.J Recept Res. 1992;12(2):149-69. doi: 10.3109/10799899209074789. J Recept Res. 1992. PMID: 1583620 Free PMC article.
Cited by
-
Adenosine A1 and A2 receptors: structure--function relationships.Med Res Rev. 1992 Sep;12(5):423-71. doi: 10.1002/med.2610120502. Med Res Rev. 1992. PMID: 1513184 Free PMC article. Review. No abstract available.
-
Molecular modeling of adenosine receptors. The ligand binding site on the rat adenosine A2A receptor.Eur J Pharmacol. 1994 Jun 15;268(1):95-104. doi: 10.1016/0922-4106(94)90124-4. Eur J Pharmacol. 1994. PMID: 7925617 Free PMC article.
-
Application of the functionalized congener approach to dendrimer-based signaling agents acting through A(2A) adenosine receptors.Purinergic Signal. 2009 Mar;5(1):39-50. doi: 10.1007/s11302-008-9113-3. Epub 2008 Jul 4. Purinergic Signal. 2009. PMID: 18600474 Free PMC article.
-
Molecular probes for the human adenosine receptors.Purinergic Signal. 2021 Mar;17(1):85-108. doi: 10.1007/s11302-020-09753-8. Epub 2020 Dec 12. Purinergic Signal. 2021. PMID: 33313997 Free PMC article. Review.
-
Chemical Probes for the Adenosine Receptors.Pharmaceuticals (Basel). 2019 Nov 12;12(4):168. doi: 10.3390/ph12040168. Pharmaceuticals (Basel). 2019. PMID: 31726680 Free PMC article. Review.
References
-
- Williams M, editor. Adenosine Receptors. Humana Press; Clifton, NJ: 1990.
-
- Olsson RA, Pearson JD. Cardiovascular purinoceptors. Physiol. Rev. 1990;70:761–845. - PubMed
-
- Lohse MJ, Elger B, Lindenborn-Fotinos J, Klotz KN, Schwabe U. Separation of solubilized A2 adenosine receptors of human platelets from non-receptor [3H]NECA binding sites by gel filtration. Naunyn-Schmiede-bergs Arch. Pharmacol. 1988;337:64–68. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
