Differential direct effects of cyclo-oxygenase-1/2 inhibition on proteoglycan turnover of human osteoarthritic cartilage: an in vitro study
- PMID: 16356188
- PMCID: PMC1526565
- DOI: 10.1186/ar1846
Differential direct effects of cyclo-oxygenase-1/2 inhibition on proteoglycan turnover of human osteoarthritic cartilage: an in vitro study
Abstract
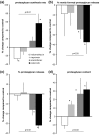
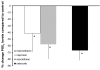
Treatment of osteoarthritis (OA) with nonsteroidal anti-inflammatory drugs (NSAIDs) diminishes inflammation along with mediators of cartilage destruction. However, NSAIDs may exert adverse direct effects on cartilage, particularly if treatment is prolonged. We therefore compared the direct effects of indomethacin, naproxen, aceclofenac and celecoxib on matrix turnover in human OA cartilage tissue. Human clinically defined OA cartilage from five different donors was exposed for 7 days in culture to indomethacin, naproxen, aceclofenac and celecoxib--agents chosen based on their cyclo-oxygenase (COX)-2 selectivity. As a control, SC-560 (a selective COX-1 inhibitor) was used. Changes in cartilage proteoglycan turnover and prostaglandin E2 production were determined. OA cartilage exhibited characteristic proteoglycan turnover. Indomethacin further inhibited proteoglycan synthesis; no significant effect of indomethacin on proteoglycan release was found, and proteoglycan content tended to decrease. Naproxen treatment was not associated with changes in any parameter. In contrast, aceclofenac and, prominently, celecoxib had beneficial effects on OA cartilage. Both were associated with increased proteoglycan synthesis and normalized release. Importantly, both NSAIDs improved proteoglycan content. Inhibition of prostaglandin E2 production indirectly showed that all NSAIDs inhibited COX, with the more COX-2 specific agents having more pronounced effects. Selective COX-1 inhibition resulted in adverse effects on all parameters, and prostaglandin E2 production was only mildly inhibited. NSAIDs with low COX-2/COX-1 selectivity exhibit adverse direct effects on OA cartilage, whereas high COX-2/COX-1 selective NSAIDs did not show such effects and might even have cartilage reparative properties.
Figures


References
-
- Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberg MC, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000;343:1520–1528. doi: 10.1056/NEJM200011233432103. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

