Future direction of renal positron emission tomography
- PMID: 16356795
- PMCID: PMC1479802
- DOI: 10.1053/j.semnuclmed.2005.08.003
Future direction of renal positron emission tomography
Abstract
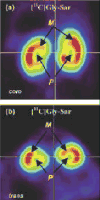
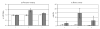
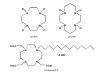
Positron emission tomography (PET) is perfectly suited for quantitative imaging of the kidneys, and the recent improvements in detector technology, computer hardware, and image processing software add to its appeal. Multiple positron emitting radioisotopes can be used for renal imaging. Some, including carbon-11, nitrogen-13, and oxygen-15, can be used at institutions with an on-site cyclotron. Other radioisotopes that may be even more useful in a clinical setting are those that either can be obtained from radionuclide generators (rubidium-82, copper-62) or have a sufficiently long half-life for transportation (fluorine-18). The clinical use of functional renal PET studies (blood flow, glomerular filtration rate) has been slow, in part because of the success of concurrent technologies, including single-photon emission computed tomography (SPECT) and planar gamma camera imaging. Renal blood flow studies can be performed with O-15-labeled water, N-13-labeled ammonia, rubidium-82, and copper-labeled PTSM. With these tracers, renal blood flow can be quantified using a modified microsphere kinetic model. Glomerular filtration can be imaged and quantified with gallium-68 EDTA or cobalt-55 EDTA. Measurements of renal blood flow with PET have potential applications in renovascular disease, in transplant rejection or acute tubular necrosis, in drug-induced nephropathies, ureteral obstruction, before and after revascularization, and before and after the placement of ureteral stents. The most important clinical application for imaging glomerular function with PET would be renovascular hypertension. Molecular imaging of the kidneys with PET is rather limited. At present, research is focused on the investigation of metabolism (acetate), membrane transporters (organic cation and anion transporters, pepT1 and pepT2, GLUT, SGLT), enzymes (ACE), and receptors (AT1R). Because many nephrological and urological disorders are initiated at the molecular and organelle levels and may remain localized at their origin for an extended period of time, new disease-specific molecular probes for PET studies of the kidneys need to be developed. Future applications of molecular renal imaging are likely to involve studies of tissue hypoxia and apoptosis in renovascular renal disease, renal cancer, and obstructive nephropathy, monitoring the molecular signatures of atherosclerotic plaques, measuring endothelial dysfunction and response to balloon revascularization and restenosis, molecular assessment of the nephrotoxic effects of cyclosporine, anticancer drugs, and radiation therapy. New radioligands will enhance the staging and follow-up of renal and prostate cancer. Methods will be developed for investigation of the kinetics of drug-delivery systems and delivery and deposition of prodrugs, reporter gene technology, delivery of gene therapy (nuclear and mitochondrial), assessment of the delivery of cellular, viral, and nonviral vectors (liposomes, polycations, fusion proteins, electroporation, hematopoietic stems cells). Of particular importance will be investigations of stem cell kinetics, including local presence, bloodborne migration, activation, seeding, and its role in renal remodeling (psychological, pathological, and therapy induced). Methods also could be established for investigating the role of receptors and oncoproteins in cellular proliferation, apoptosis, tubular atrophy, and interstitial fibrosis; monitoring ras gene targeting in kidney diseases, assessing cell therapy devices (bioartificial filters, renal tubule assist devices, and bioarticial kidneys), and targeting of signal transduction moleculas with growth factors and cytokines. These potential new approaches are, at best, in an experimental stage, and more research will be needed for their implementation.
Figures


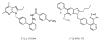
References
-
- Hewitt SM, Dear J, Star RA. Discovery of protein biomarkers for renal diseases. J Am Soc Nephrol. 2004;15:1677–1689. - PubMed
-
- Lorenz CH, Powers TA, Partain CL. Quantitative imaging of renal blood flow and function. Invest Radiol. 1992;27 (Suppl 2):S109–S114. - PubMed
-
- Romero JC, Lerman LO. Novel noninvasive techniques for studying renal function in man. Semin Nephrol. 2000;20:456–462. - PubMed
-
- Townsend DW. Physical principles and technology of clinical PET imaging. Ann Acad Med Singapore. 2004;33:133–145. - PubMed
-
- Judenhofer MS, Pichler BJ, Cherry SR. Evaluation of high performance data acquisition boards for simultaneous sampling of fast signals from PET detectors. Physics in Medicine and Biology. 2005;50:29–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

